|
 |
| jbm > Volume 26(2); 2019 > Article |
|
Abstract
Background
The objective of the current study is to determine the role of serum parathyroid hormone (PTH) on hip fracture development by retrospectively analyzing the relationship between vitamin D and PTH levels and hip fracture prevalence.
Methods
Among 288 patients over 50 years of age, 113 patients with hip fracture and 111 controls without fracture were analyzed after excluding patients with conditions affecting bone metabolism. Bone mineral density and serum biochemical markers were measured, while demographic data were obtained. Patients were divided into 4 groups according to serum 25-hydroxy-vitamin D (25-[OH]D) and PTH levels: LowD+LowP (low 25[OH]D and PTH); LowD+HighP, (low 25[OH]D and high PTH); HighD+LowP (high 25[OH]D and low PTH); and HighD+HighP, patients with (high 25[OH]D and PTH). Measured values and percentages of patients with hip fracture in each group were then determined and compared.
Results
The number of patients included in the LowD+LowP, LowD+HighP, HighD+LowP, and HighD+HighP groups was 116, 17, 87, and 4, while the percentages of patients with hip fracture in the same groups were 60.3%, 88.2%, 27.6%, and 100%, respectively. The percentage of hip fracture was significantly lower in the LowD+LowP than the LowD+HighP group (P=0.049).
Conclusions
Patients with low serum 25(OH)D and PTH levels showed lower hip fracture prevalence, indicating the potential protective role of low PTH levels on bone health in patients with vitamin D deficiency. Therefore, clinicians should pay more attention to the possibility of fractures in patients with vitamin D deficiency who present with high PTH levels.
The homeostasis of calcium metabolism is closely related to bone health. Thus, key regulators of calcium metabolism, vitamin D, and parathyroid hormone (PTH) are strongly associated with bone health.[1,2,3,4] Moreover, vitamin D deficiency leads to poor bone mineral density (BMD) and increases the risk for fractures.[5,6,7] A high prevalence of vitamin D deficiency has been reported,[8,9] and the incidence of osteoporotic hip fractures has been increasing in South Korea.[10]
Vitamin D deficiency stimulates PTH secretion that facilitates bone resorption.[11,12] Therefore, several studies have shown that BMD is positively associated with serum 25-hydroxy-vitamin D (25-[OH]D) [2,13,14,15] and negatively correlated with serum PTH levels.[4,16,17] Several studies have also shown that patients with hip fracture have significantly lower serum 25(OH)D and higher PTH levels than controls.[18,19,20,21]
Nonetheless, Sahota et al.[22] reported the presence of functional hypoparathyroidism, a state in which serum PTH levels do not increase despite vitamin D deficiency, while noting the protective effects of low PTH levels on the bone, even with low serum 25(OH)D levels.[4] This report showed that among patients with vitamin D deficiency, those with normal PTH showed higher BMDs than those with secondary hyperparathyroidism. On the other hand, some studies in patients with vitamin D deficiency have reported that vitamin D is an independent risk factor for fractures and that PTH is not associated with fracture risk. [23,24] Collectively, the effects of functional hypoparathyroidism on bone health remain controversial.
We hypothesized that patients with functional hypoparathyroidism would show lower prevalence of hip fractures compared to vitamin D deficient patients with increased PTH level.
Therefore, the purpose of this study was to determine the correlation of low serum PTH levels with hip fracture by analyzing the relationship between vitamin D and PTH status and hip fracture prevalence in Korean patients over 50 years old.
This study was conducted after obtaining approval from the Institutional Review Board (IRB) of Asan Medical Center (IRB No. 2015-0279). The present study initially screened 288 patients over 50 years of age who were admitted for hip surgery from February 2011 to December 2013. Patients with a history of osteoporosis treatment (16 patients) or with systemic diseases (24 patients), such as malignancy, chronic renal failure (a glomerular filtration rate of less than 30 mL/min/1.73 m2), and thyroid disease, and those using steroids (21 patients) because of organ transplant or autoimmune diseases, such as systemic lupus erythematous and rheumatoid arthritis, were excluded from this study. Patients with a history of previous hip fracture (3 patients) were also excluded from the analysis. A total of 224 patients were finally included, and there were 113 had hip fracture and 111 controls (patients with osteoarthritis or osteonecrosis). A flowchart of the patients included in this study is presented in Figure 1.
Vitamin D deficiency was defined as serum 25(OH)D levels below 15.1 ng/mL. The cut-off value of PTH was 64 pg/mL and was determined following previous studies on functional parathyroidism.[25] Consequently, patients were divided into 4 groups according to serum 25(OH)D and PTH levels: (1) LowD+LowP, patients with low serum 25(OH)D and PTH levels; (2) LowD+HighP, patients with low serum 25(OH)D and high serum PTH levels; (3) HighD+LowP, patients with high serum 25(OH)D and low serum PTH levels; and (4) HighD+HighP, patients with high serum 25(OH)D and PTH levels.
To assess calcium homeostasis in patients, biochemical markers including PTH, 25(OH)D, osteocalcin, C-terminal telopeptide of type I collagen (CTX), and ionized calcium were evaluated. To measure biochemical markers, blood samples were obtained after fasting and subsequently analyzed at a certified laboratory in our institution. Blood was collected the morning after admission following an overnight fast.
Serum intact PTH levels were measured using a standard enzyme-linked immunosorbent assay-PTH immunoradiometric assay (IBL International GmbH, Hamburg, Germany) with 1.0 pg/mL as the lower limit of detection. Intra- and interassay coefficients of variation (CVs) were below 5%. Serum concentrations of 25(OH)D were measured through radioimmunoassay using a 25(OH)D3-specific kit (Cobra II Auto-γ Counting System, Packard Instruments, Downers Grove, IL, USA). Intra- and interassay CVs for these analyses were consistently <3.5%. Serum CTX concentrations were measured using an electrochemical luminescence immunoassay (Roche Diagnostics, Mannheim, Germany) with intra- and interassay CVs of 1.0% to 4.6% and 1.6% to 4.7%, respectively. Serum osteocalcin concentrations were also measured using an electrochemical luminescence immunoassay (Roche Diagnostics) with intra- and interassay CVs of 1.2% to 4.0% and 1.7% to 6.5%, respectively. Serum levels of ionized calcium were measured from arterial blood using a Stat Profile® Critical Care Xpress analyzer (Nova Biomedical, Waltham, MA, USA).
Areal BMD (g/cm2) was measured at the lumbar spine (L1-L4) and proximal femur (femur neck and total hip) at a tertiary medical center using dual energy X-ray absorptiometry (Lunar Prodigy Advance; GE Lunar, Madison, WI, USA) with software version 9.30.044. The precision of this equipment, which is determined through CV, was 0.67% and 1.25% for the lumbar spine and femoral neck, respectively, according to another study conducted on 17 volunteers at the same institution.[26] Demographic data, including age, body mass index (BMI), and sex was also collected.
All continuous variables were tested for normality using the Shapiro-Wilk test. Measurements were expressed as means±standard deviations with 95% confidence intervals for continuous variables. Comparisons among the 4 groups (quadrants) according to continuous variables (e.g., 25[OH]D and PTH levels) were made using analysis of variance or Kruskal-Wallis (if normality was not assumed). Comparisons according to discrete variables (e.g., sex) were made using the χ2 test. Logistic regression analysis was performed to determine the relationship between serum 25(OH)D levels and the probability of fracture in all included patients. The logistic regression coefficient was statistically significant. The model was assessed for goodness of fit using the Hosmer and Lemeshow test, which showed that the model was well specified and fitted the data. The regression equation was as follows:
In this regression equation, the 25(OH)D level that satisfies “prob (no fracture)=prob (fracture)=0.5” was 15.1, which means that a patient with a serum 25(OH)D level of 15.1 has a 50:50 probability of having hip fracture or disease. For this reason, 15.1 was set as the cut-off value for 25(OH) D in this study. A P-value of less than 0.05 was considered significant. Statistical software MedCalc (version 11.6; Med-Calc software, Mariakerke, Belgium) and R (version 3.1.0; The R Foundation for Statistical Computing, Vienna, Austria) had been used for all statistical analyses.
The proportion of patients with fracture in the LowD+LowP, LowD+HighP, HighD+LowP, and HighD+HighP groups was 60.3% (70/116), 88.2% (15/17), 27.6% (24/87), and 100% (4/4), respectively. The LowD+highP group showed a significantly higher prevalence of fractures than the HighD+LowP and LowD+LowP groups (P<0.001 and P=0.049, respectively; Fig. 2).
A total of 116 patients, comprising 51.8% of the study population and 87.2% of patients with low serum 25(OH)D, were included in the LowD+LowP group, which represents functional hypoparathyroidism.
Regarding biochemical measurements, serum levels of CTX and ionized calcium showed significant difference between the LowD+HighP and HighD+LowP group (P=0.017 and 0.027, respectively). The values of osteocalcin level were not significantly different among the groups.
Total hip BMD values in the LowD+LowP, LowD+HighP, HighD+LowP, and HighD+HighP groups were 0.754±0.201, 0.681±0.151, 0.876±0.148, and 0.593±0.091, while spine BMD values in the same groups were 0.932±0.227, 0.847±0.184, 1.020±0.191, and 0.673±0.181, respectively. The HighD+LowP group had significantly higher total hip and spine BMDs than the other subgroups (P<0.001 in all).
No significant differences in age, sex ratio, ionized calcium, osteocalcin, CTX levels, and BMD were found between the LowD+LowP and LowD+HighP groups. The LowD+HighP group had a significantly lower BMI than the LowD+LowP group (P=0.002). Demographic data and estimated mean values of the variables in each group with statistical analyses are presented in Table 1.
In the present study, we divided the patients into 4 subgroups according to their serum 25(OH)D and PTH levels to evaluate the effect of serum PTH on hip fracture risk, especially in patients with vitamin D deficiency. Among patients with vitamin D deficiency, those with low serum PTH levels (LowD+LowP) showed a significantly lower prevalence of hip fracture than those with high serum PTH levels (LowD+HighP). This result suggests that low serum PTH levels may have a protective effect on bone health in patients with vitamin D deficiency.
The osteoclastogenic action of PTH through RANKL stimulation has been well established. Moreover, high serum PTH levels have been reported to be associated with increased bone turnover and decreased bone mass.[12,27,28] Sahota et al.[4] previously reported that among patients with vitamin D deficiency, those with low serum PTH levels had significantly higher BMD than those with high serum PTH levels, suggesting that functional hypoparathyroidism has a beneficial effect on bone health. Similarly, the current study showed that among patients with vitamin D deficiency, those with high serum PTH levels (LowD+HighP) had lower mean hip and spine BMD values and higher serum CTX levels than those with low serum PTH levels (LowD+LowP), although the difference was not statistically significant. We believe that the statistically insignificant results might have been caused by the small number of subjects in the LowD+HighP group.
More important than bone metabolism or BMD are fractures that affect patients' functional status and survival. This study found that among patients with vitamin D deficiency, low serum PTH levels (LowD+LowP) led to lower hip fracture prevalence than high serum PTH levels (LowD+HighP). Previous reports have also reported about the effects of serum PTH on hip fracture. Dhanwal et al.[19] showed that two-thirds of patients with hip fracture had secondary hyperparathyroidism. Rejnmark et al.[29] also reported that high levels of PTH are related to high risk for fractures in patients with low vitamin D levels. In addition to the osteoclastogenic effect of PTH, increased risk for falls resulting from impaired muscle function associated with high serum PTH may also be contributory.[30,31,32]
We note that reports with contrary results regarding the effect of functional hypoparathyroidism on bone health do exist. Amouzougan et al.[25] found no significant difference in hip and spine BMD among patients with vitamin D deficiency who had high and low serum PTH levels. We believe that this contrasting result was caused by the difference in the criterion for vitamin D deficiency. In their analysis, the criterion of vitamin D deficiency was 30 ng/mL. Other studies (including the present study) have shown that the beneficial effects of functional hypoparathyroidism become apparent when using a criterion for vitamin D deficiency that is much lower than 30 ng/mL.[4] These results suggest that the effects of PTH may be amplified when analyzing patients who have vitamin D concentrations lower than 15 ng/mL. Notably, Yamauchi et al.[24] had shown that functional hypoparathyroidism was a risk factor for fragility fractures. However, their analysis compared fracture risk between patients with functional hypoparathyroidism and those with sufficient vitamin D levels, not secondary hyperparathyroidism (LowD+HighP). Therefore, the effects of PTH levels on fracture risk cannot be confirmed from their results.
The present study showed that 87.2% of those with vitamin D deficiency had normal serum PTH levels despite low serum 25(OH)D values. Previous studies have reported that 40% to 88% of subjects with vitamin D deficiency showed normal serum PTH values.[4,21,24,33] Different criteria for vitamin deficiency and hyperparathyrodism among studies may have caused these differing results. Using statistical analysis of fracture risk, the current analyses defined vitamin D deficiency as a 25(OH)D level lower than 15.1 ng/mL. Although this cut-off value was lower than that suggested by other authors,[34,35,36] previous reports also showed that a 25(OH)D level lower than 15 ng/mL was related to increased fracture risk or decreased BMD.[23,25] Nevertheless, establishing the ideal 25(OH)D level was beyond the scope of the present study, and the cut-off value we adopted was suitable for comparing fracture risk according to PTH values.
The very high prevalence of functional hypoparathyroidism in the current study may have been caused by the characteristics of the study population. The current study included not only patients with osteoporotic fracture but also those without osteoporosis.
The present study found that patients with LowD+HighP had significantly lower BMI than those with LowD+LowP. This result seems to be contrary to that of previous findings wherein BMI was positively associated with serum PTH levels.[27,28] However, considering that the mean BMI of the LowD+LowP group (24.2±3.7 kg/m2) was within the normal range and that elderly patients were included in this study, the higher BMI of the LowD+LowP group may reflect a better nutritional state, including calcium intake, compared to the LowD+HighP group.
This study had some limitations. First, the cross-sectional design of this study did not allow the detection of causal relationships. Second, because of its retrospective design, concerns may be raised regarding bias in data collection. However, although the analysis was performed retrospectively, materials in the current study were collected prospectively. Therefore, these concerns may be overlooked to some extent. Third, the number of patients in the HighD+HighP was only 4, and this can be a cause of type 2 error. Finally, given that the control group did not contain healthy patients, care should be taken when interpreting the results of the current study.
In conclusion, this study highlights a potential protective effect of low PTH levels on bone health in patients with vitamin D deficiency. We recommend that clinicians pay more attention to the possibility of fracture in patients with vitamin D deficiency who present with high PTH levels.
References
1. Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 2001;22:477-501.


2. Kuchuk NO, Pluijm SM, van Schoor NM, et al. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab 2009;94:1244-1250.


3. Rejnmark L, Vestergaard P, Brot C, et al. Parathyroid response to vitamin D insufficiency: relations to bone, body composition and to lifestyle characteristics. Clin Endocrinol (Oxf) 2008;69:29-35.


4. Sahota O, Mundey MK, San P, et al. The relationship between vitamin D and parathyroid hormone: calcium homeostasis, bone turnover, and bone mineral density in postmenopausal women with established osteoporosis. Bone 2004;35:312-319.


5. Chapuy MC, Schott AM, Garnero P, et al. EPIDOS Study Group. Healthy elderly French women living at home have secondary hyperparathyroidism and high bone turnover in winter. J Clin Endocrinol Metab 1996;81:1129-1133.

6. Bakhtiyarova S, Lesnyak O, Kyznesova N, et al. Vitamin D status among patients with hip fracture and elderly control subjects in Yekaterinburg, Russia. Osteoporos Int 2006;17:441-446.


8. Bi X, Tey SL, Leong C, et al. Prevalence of vitamin D deficiency in Singapore: Its implications to cardiovascular risk factors. PLoS One 2016;11:e0147616.



9. Jung IK. Prevalence of vitamin D deficiency in Korea: Results from KNHANES 2010 to 2011. J Nutr Health 2013;46:540-551.

10. Choi ES, Shon HC, Kim YM, et al. Is the incidence rate of hip fractures still increasing in Korea?: an epidemiologic study based on national health insurance database. J Korean Orthop Assoc 2016;51:447-454.

13. Bischoff-Ferrari HA, Kiel DP, Dawson-Hughes B, et al. Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. J Bone Miner Res 2009;24:935-942.


14. Hannan MT, Litman HJ, Araujo AB, et al. Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab 2008;93:40-46.


15. Kuchuk NO, van Schoor NM, Pluijm SM, et al. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: global perspective. J Bone Miner Res 2009;24:693-701.


16. Hagström E, Lundgren E, Mallmin H, et al. Positive effect of parathyroidectomy on bone mineral density in mild asymptomatic primary hyperparathyroidism. J Intern Med 2006;259:191-198.


17. Jorde R, Sundsfjord J. Bone mineral density and blood pressure in patients with asymptomatic hyperparathyroidism. The Tromso Study. J Intern Med 2000;247:325-330.


18. Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med 1995;332:767-773.


19. Dhanwal DK, Sahoo S, Gautam VK, et al. Hip fracture patients in India have vitamin D deficiency and secondary hyperparathyroidism. Osteoporos Int 2013;24:553-557.


20. Larrosa M, Gomez A, Casado E, et al. Hypovitaminosis D as a risk factor of hip fracture severity. Osteoporos Int 2012;23:607-614.


21. Sakuma M, Endo N, Oinuma T, et al. Vitamin D and intact PTH status in patients with hip fracture. Osteoporos Int 2006;17:1608-1614.

22. Sahota O, Gaynor K, Harwood RH, et al. Hypovitaminosis D and ‘functional hypoparathyroidism’-the NoNoF (Nottingham Neck of Femur) study. Age Ageing 2001;30:467-472.


23. Melhus H, Snellman G, Gedeborg R, et al. Plasma 25-hydroxyvitamin D levels and fracture risk in a community-based cohort of elderly men in Sweden. J Clin Endocrinol Metab 2010;95:2637-2645.


24. Yamauchi M, Kaji H, Nawata K, et al. Role of parathyroid hormone in bone fragility of postmenopausal women with vitamin D insufficiency. Calcif Tissue Int 2011;88:362-369.


25. Amouzougan A, Chopin F, Laporte S, et al. Functional hypoparathyroidism in postmenopausal women with fragility fracture. Joint Bone Spine 2012;79:170-175.


26. Kim H, Ahn SH, Shin C, et al. The association of higher plasma macrophage migration inhibitory factor levels with lower bone mineral density and higher bone turnover rate in postmenopausal women. Endocrinol Metab (Seoul) 2016;31:454-461.



27. Lips P, Duong T, Oleksik A, et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab 2001;86:1212-1221.

29. Rejnmark L, Vestergaard P, Brot C, et al. Increased fracture risk in normocalcemic postmenopausal women with high parathyroid hormone levels: a 16-year follow-up study. Calcif Tissue Int 2011;88:238-245.


30. Sikjaer T, Rolighed L, Hess A, et al. Effects of PTH(1-84) therapy on muscle function and quality of life in hypoparathyroidism: results from a randomized controlled trial. Osteoporos Int 2014;25:1717-1726.


31. Gielen E, O'Neill TW, Pye SR, et al. Endocrine determinants of incident sarcopenia in middle-aged and elderly European men. J Cachexia Sarcopenia Muscle 2015;6:242-252.



32. Renoud A, Ecochard R, Marchand F, et al. Predictive parameters of accelerated muscle loss in men-MINOS study. Am J Med 2014;127:554-561.


33. Serhan E, Newton P, Ali HA, et al. Prevalence of hypovitaminosis D in Indo-Asian patients attending a rheumatology clinic. Bone 1999;25:609-611.

34. Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006;81:353-373.


Fig. 1
A flow chart of the retrospective analysis. This retrospective study was conducted by enrolling 288 patients who underwent hip surgery between 2011 and 2013. After excluding those unsuitable for analysis, 224 patients were ultimately included in this study. Patients were divided into 4 subgroups according to serum 25-hydroxy-vitamin D (25[OH]D) 3 and parathyroid hormone (PTH) levels.

Fig. 2
Scatter diagram showing the proportion of hip fractures. The circle indicates the control group, while the orange square indicates patients with hip fracture. The low 25-hydroxy-vitamin D (25[OH]D) and high parathyroid hormone (PTH) group showed a higher percentage of fracture patients than the low 25(OH)D and low PTH group.
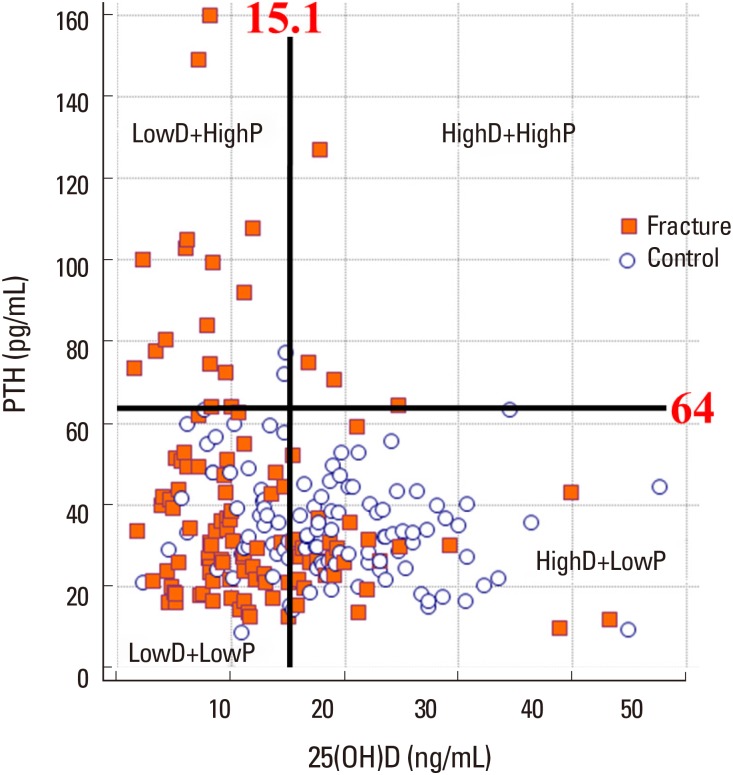
Table 1
Characteristics, hip fracture ratio and measurements of each group divided by 25-hydroxy-vitamin D and parathyroid hormone level
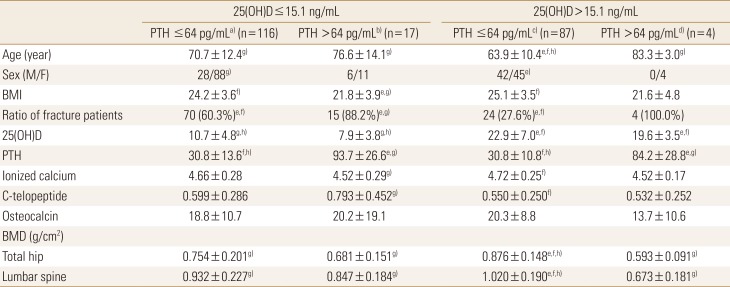
The data is presented as number (%) or mean±standard deviation.
a)Low 25(OH)D and low PTH. b)Low 25(OH)D and high PTH. c)High 25(OH)D and low PTH. d)High 25(OH)D and high PTH. e)P<0.05, vs. subgroup low 25(OH)D and low PTH. f)P<0.05, vs. subgroup low 25(OH)D and high PTH. g)P<0.05, vs. subgroup high 25(OH)D and low PTH. h)P<0.05, vs. subgroup high 25(OH)D and high PTH.
M, male; F, female; BMI, body mass index; 25(OH)D, 25-hydroxy-vitamin D; PTH, parathyroid hormone; BMD, bone mineral density




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print