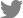|
 |
| jbm > Volume 30(4); 2023 > Article |
|
Abstract
Background
Treating osteoporosis in patients with a distal radius fracture (DRF) became paramount at the Fracture Liaison Service. Spinal sagittal imbalance emerged as a risk factor for subsequent fractures. Therefore, here we investigated the spinal profile of patients with DRF to investigate its association with a history of falls and prevalent vertebral fractures.
Methods
We reviewed the cases of 162 women presenting with DRF and 162 age-matched women without fracture who underwent an osteoporosis evaluation including bone mineral density (BMD) and lateral spine imaging. We compared the incidence of prevalent vertebral fracture and sagittal vertical axis (SVA) to measure spinal sagittal imbalance. We also performed a regression analysis of the risks of prevalent vertebral fracture, such as age, body mass index (BMI), BMD, and SVA.
Results
The SVA was significantly smaller (indicating more stable sagittal balance) in patients with a DRF versus controls (16 mm vs. 34 mm, respectively; P<0.001). The incidence of a prevalent vertebral fracture was similar between groups (12% vs. 15%, respectively; P=0.332). In both groups, the SVA was significantly greater in those with versus without a vertebral fracture. The vertebral fracture was significantly associated with age and SVA but not BMI or spinal BMD.
Distal radius fracture (DRF) is the most common upper extremity fracture.[1] Since DRF patients are at a higher risk for subsequent fracture,[2] they are a good target for osteoporosis prevention. A previous study has shown that active osteoporosis care of DRF patients significantly reduces subsequent fractures.[3] Physicians now have a wide range of osteoporosis medications, enabling more individualized therapies, such as a “treat-to-target” approach.[4,5] Therefore, identification of additional risk factors in patients presenting with a DRF would be helpful for this paradigm shift.
The vertebral column is the most common site for fragility fractures and is a strong predictor for subsequent fractures.[6-8] Two-third of prevalent vertebral fractures are underdiagnosed, and they are found in 20% to 26% of Fracture Liaison Service patients.[6] However, their prevalence has not been specifically studied in patients presenting with DRF. Another useful indicator for subsequent fracture actively being investigated is the sagittal vertical axis (SVA) measurement. This represents the amount of spinal sagittal imbalance. Kaneko et al. [9] reported that the SVA is significantly greater in DRF patients compared to a normal group, suggesting that increased SVA leads to a higher risk of falling. Lin et al. [10], published a study showing that the SVA is the most important independent risk factor for vertebral fractures. They proposed that spinal imbalance destroys biomechanical stability. This theory was investigated through a finite element model, and vertebral strength decreased as posture fell into sagittal imbalance.[11] Recently, Asahi et al. [12], also reported that SVA increases osteoporosis-related fractures with odds ratios (OR) of 4.228. Therefore, we thought that the investigation of spinal sagittal imbalance in DRF patients becomes very important.
In this study, we evaluated and compared spinal sagittal imbalances and prevalent vertebral fractures in a group of DRF patients to a group of age-matched controls without a definite history of other fractures or falls. We wanted to know whether a spinal sagittal imbalance attributed to falling can cause DRF or correlate with prevalent vertebral fractures.
This study was performed in a single referral training hospital, and we obtained approval for this study from the Institutional Review Board. This study was a cross-sectional, retrospective review of medical records, so we were given an exemption for participants’ written consent. We investigated the cases of female patients aged 55 or older who had received treatment for DRF due to fall, either operatively or nonoperatively, at our institute from January 2016 to August 2021. We included patients with records of bone densitometry and lateral spine plain radiographs. For the control group, we reviewed female patients of the same age who had visited the orthopedic clinic of our hospital during the same period for treatment of locomotive syndrome and had the same exam, but we excluded those women who had undergone treatment for spinal diseases or those with a definite history of falls or fractures. The history of falls or trauma is routinely recorded at the first visit to our orthopedic department, so we used these records during the chart review. Especially for those with prevalent vertebral fractures, we double-checked whether they had symptoms and referred them to the spine clinic for treatment. We also excluded them if any were identified. We used the Clinical Data Warehouse (CDW) at our institute to find the DRF and control participants who met the criteria. Out of 163 DRF cases, we excluded one because of a hip fracture history. We also utilized the CDW to screen 952 controls, followed by the exclusion process and the propensity score matching, to find age-matched 162 controls.
We obtained age, body mass index (BMI) in kg/m2, and bone mineral density (BMD) in g/cm2 of the spine, femur neck, and total hip with dual energy X-ray absorptiometry (DXA) from certified radiological technologists using a single DXA scanner (Discovery W; Hologic Inc., Bedford, MA, USA). We did a radiographic evaluation of the SVA (in mm), and the presence of a prevalent vertebral fracture. We obtain the SVA measurements using our picture archiving and communication system’s ruler and functions for drawing lines. A C7 plumb line was drawn at the center of the C7 vertebra. The horizontal distance between this line and the posterior superior corner of the S1 vertebra was measured with the ruler function (Fig. 1). We identified prevalent vertebral fractures by direct visualization suggested by the Genant semiquantitative grading scale.[13] A vertebral body was considered to be fractured if mild deformation (approximately 20%-25% reduction in the anterior, middle, or posterior height and a reduction of area 10%-20% or worse) was present. Worse deformities were also categorized into moderate (25%-40% reduction in height) and severe (40% reduction in height). Genant’s method was also applied to classify the shape of prevalent vertebral fractures into a biconcave, wedge, or crush shape (Fig. 2). Board-certified radiologists at our institute also identified and confirmed all vertebral fractures. We also measured the Cobb angle of prevalent vertebral fracture in the sagittal plane. If multiple prevalent vertebral fractures were present in one subject, the greatest Cobb angle was recorded.
The parameters listed above were compared between the 2 groups. Before the analysis, we attempted propensity score matching based on a logistic regression analysis using age. After matching, the standardized mean age difference was below 0.1. Correlations between parameters were analyzed with Pearson’s correlation coefficient. The SVAs of controls and cases were compared using the t-test. We performed cross-tabulation using Pearson χ2 to find the association between the occurrence of DRF and prevalent vertebral fracture. We used logistic regression analysis to evaluate the risk factors for prevalent vertebral fractures. Variance inflation factors (VIFs) of parameters were also examined. Total hip BMD was excluded from this analysis because it correlated with femur neck BMD (correlation coefficient, 0.868) and high multicollinearity (VIF, 4.580). Multivariate analysis was finally performed. All statistical analyses were done with SPSS software (version 26.0; IBM Corp., Armonk, NY, USA), and a P value less than 0.05 was considered significant.
There were 162 DRF patients that met the inclusion and exclusion criteria. The mean age was 69 (range, 55-88). Out of 952 controls, a one-to-one age-matched propensity score produced 162 corresponding controls, and their mean age was 69 (range, 55-87).
The SVA was significantly smaller (more stable sagittal balance) in patients with a DRF than in control patients (16 mm vs. 34 mm; P<0.001) (Table 1). The patients with a DRF had significantly lower BMI and BMD, and they had a higher prevalence of osteoporosis. However, the incidence of prevalent vertebral fracture was similar between the 2 groups (12% in DRF vs.15% in control patients; P=0.332).
In both groups, the SVA was significantly greater in those with a vertebral fracture than in those without (Fig. 3); In the DRF group, the mean SVA was 59 mm in those with vertebral fracture and 10 mm in those without a fracture (P<0.001). In the control patients, it was 59 mm in those with vertebral fractures and 29 mm in those without (P= 0.001).
The SVA significantly correlated with age, femur neck BMD, absence of DRF, and presence of prevalent vertebral fracture (Table 2). A correlation between the occurrence of DRF and prevalent vertebral fractures was not found to be significant (P=0.332).
Out of 44 vertebral fractures, 31 were biconcave, 12 were wedge, and only one was crush type. Thirteen cases were moderate deformity, and 31 cases were severe deformity. The average Cobb angle was 11.2 degrees (range, −21.4 to 32.0). The correlation between the Cobb angle and SVA was insignificant (P=0.832).
In the univariate analysis, age, femur neck BMD, and SVA were all significant predictors of prevalent vertebral fracture (Table 3). Spine BMD did not correlate with prevalent vertebral fractures. Only older age and greater SVA were independently associated with prevalent vertebral fracture in the multivariate analysis (OR, 1.070, P=0.006; OR, 1.009, P=0.034, respectively).
In this study, we investigated and compared the spinal profile of patients with DRF to a group of control patients. The prevalence of vertebral fracture was similar in both groups, and the control patients showed worse sagittal imbalance (greater SVA). In both groups, those with vertebral fractures had a higher SVA. This result suggests that the sagittal imbalance is associated with prevalent vertebral fracture, yet the definite history of falls that cause DRF did not present a worse sagittal imbalance.
DRF is an early sign of general osteoporosis but also is one of the major osteoporotic fractures.[14,15] Most osteoporotic fractures, especially hip and wrist fractures, are caused by falls.[16] Thus, a fall risk assessment is very important. A DRF is usually caused by a fall on the outstretched hand.[17] Recently, this fall has been suggested to be caused by sagittal imbalance.[9] Kaneko et al. [9] showed a higher SVA in DRF patients. However, Kim et al. [18] also investigated the association between sagittal balance and the risk of falls and showed no association between the SVA and the risk of falling. Our results showed significantly lower SVA in patients with DRF. The superior sagittal balance of DRF patients has never been reported on before. This does not necessarily reflect the association between falls and SVA, but we may deduct an indirect relationship. Falling on an outstretched hand is one of the natural reflexes to falling [19] and also a protection against direct impact on the hip.[20] This reflex may be impaired as the patient ages, so wrist fractures tend to occur in persons who are in relatively good health as compared to those with hip fractures.[21] Lower SVA in patients with DRF can be understood as one of the parameters of this relatively good reflex status.
The normal upper limit of SVA is 5 cm, and even among the elderly (those older than 60) reported mean SVA is 2.2 cm.[11] Since the mean SVA for those with prevalent vertebral fracture in both groups of our study was 5.9 cm, they belong to the diseased group in terms of kyphotic deformity. As vertebral fractures themselves can destroy sagittal balance, worse sagittal imbalance has been reported in patients who suffered osteoporotic vertebral fractures.[22] Another study also showed increased T1-pelvic angle in patients with vertebral fractures.[23] In that study, the author defined vertebral fractures when vertebral deformity (Cobb angle) was equal to or greater than 10 degrees. However, our analysis of prevalent vertebral fracture showed that 70% were biconcave deformity, and half of them was less than 10 degrees. The correlation between vertebral deformity and SVA was not significant. Therefore, we think vertebral fracture does not always increase SVA. Rather, a sagittal imbalance can imply muscle weakness because paravertebral muscle and psoas are important for the maintenance of spinal alignment.[24,25] These reports are aligned with the recent emphasis made on an evaluation of sarcopenia in patients with DRF.[26] The possibility of improvement in SVA after exercise such as “locomotion training” has been suggested.[27] Therefore, such exercise may be helpful in osteoporotic patients with sagittal imbalance.
Obtaining lateral spine images has been suggested to identify prevalent vertebral fractures during osteoporosis evaluation.[28,29] A similar recommendation has recently been reported for using DXA to determine vertebral fractures.[30] The advancement of DXA instrumentation has enabled the detection of vertebral fractures. Compared to conventional lateral spine radiographs, these advantages include lower cost, lower radiation exposure, and greater convenience. However, the conventional radiograph remains the gold standard for its superior image resolution. Since degenerative changes of the spine predominate in osteoporotic patients [31] and such changes affect lumbar BMD evaluation, these should also be investigated in conventional lateral radiographs.
There are a few limitations to this study. First, this was a retrospective study. It was not a strictly controlled study. Although we excluded any controls with a history of major falls or trauma, minor fall events may have been omitted. Second, one may argue that the significantly low BMI and BMD of the DRF group contaminated the analysis. Obesity always interfered with our understanding of osteoporotic fractures. As BMI increases as BMD increases, obesity has been suggested as a protective factor against osteoporotic fractures.[32] However, this has been recently refuted.[33] Obesity in wrist fractures is especially in the debate.[33-36] Therefore, a similar prevalence of vertebral fracture observed in both the DRF group and control group despite the difference in BMI and BMD was a meaningful finding. We can also add value to SVA because it highly correlates with cortical bone marrow densities yet it does not correlate with BMI. Last, since this is a cross-sectional study any temporal relationship to prove investigated parameters as risk factors could not be assessed. A future prospective design would provide better evidence for the association between SVA and DRF or prevalent vertebral fracture, but our study still provides a valuable background.
In conclusion, this study shows that the sagittal imbalance is associated with prevalent vertebral fractures but not falls that can lead to DRF. In addition, the association of vertebral fracture with sagittal imbalance but not with spine BMD suggests that the mechanical factor can be a more important mechanism of vertebral fracture in some patients. Identification of this unique spinal profile may help man age osteoporosis in patients presenting with DRF.
Acknowledgments
The following manuscript was proofread and edited by the professional English editors at HARRISCO.
DECLARATIONS
Funding
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (Grant No. RS-2023-00209251).
Fig. 1
Measurement of sagittal vertical axis shown with exemplary case and control. C7 plumb line was drawn at the center of the C7 vertebra. The horizontal distance between this line and the posterior superior corner of the S1 vertebra was measured.

Fig. 2
Genant’s classification on the shape of deformity. Cobb angle of the fractured vertebral body that caused the greatest deformity was measured as shown in the lower right capture.
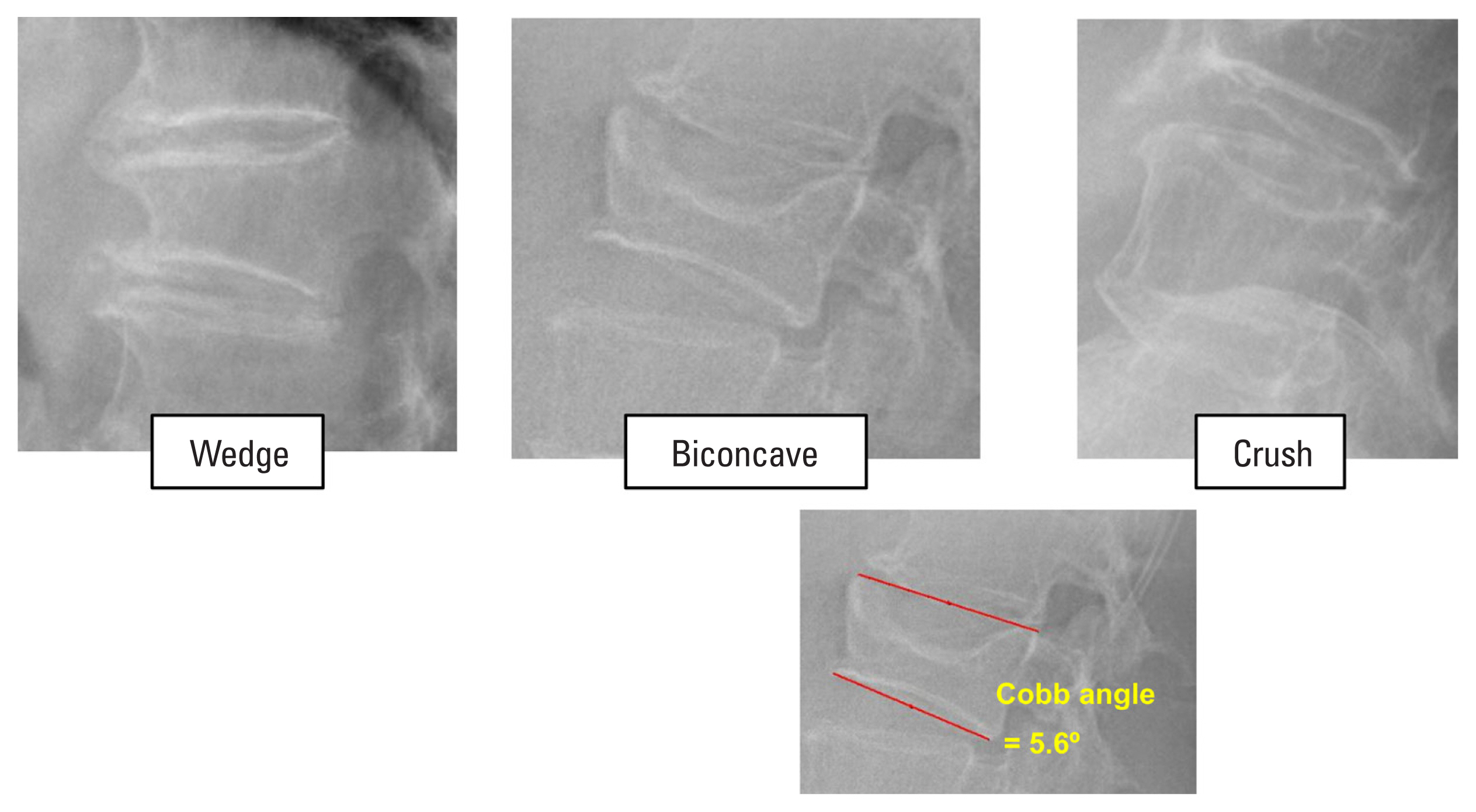
Fig. 3
Box and whisker diagram of sagittal vertical axis of control and case subgrouped on the presence of vertebral fracture.

Table 1
Comparison of parameters between case and control
Table 2
Correlation coefficient among parameters
| Age | BMI | SVA | Spine BMD | FN BMD | TH BMD | DRF | PVF | |
|---|---|---|---|---|---|---|---|---|
| Age | 1 | −0.100 (P=0.087) | 0.508a) (P<0.001) | −0.750 (P=0.181) | −0.434a) (P<0.001) | −0.414a) (P<0.001) | −0.002 (P=0.971) | 0.326a) (P<0.001) |
| BMI | 1 | 0.029 (P=0.614) | 0.362a) (P<0.001) | 0.324a) (P<0.001) | 0.377a) (P<0.001) | −0.431a) (P<0.001) | −0.028 (P=0.637) | |
| SVA | 1 | 0.018 (P<0.748) | −0.267a) (P<0.001) | −0.276a) (P<0.001) | −0.196a) (P<0.001) | 0.298a) (P<0.001) | ||
| Spine BMD | 1 | 0.501a) (P<0.001) | 0.531a) (P<0.001) | −0.268a) (P<0.001) | −0.021 (P=0.709) | |||
| FN BMD | 1 | 0.868a,b) (P<0.001) | −0.149a) (P=0.007) | −0.229a) (P<0.001) | ||||
| TH BMD | 1 | −0.119a) (P=0.033) | −0.237a) (P<0.001) | |||||
| DRF | 1 | −0.054 (P=0.332) | ||||||
| PVF | 1 |
Table 3
OR of parameters for the occurrence of vertebral fracture
REFERENCES
1. Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 2007;22:465-75.
https://doi.org/10.1359/jbmr.061113.


2. Crandall CJ, Hovey KM, Cauley JA, et al. Wrist fracture and risk of subsequent fracture: findings from the women’s health initiative study. J Bone Miner Res 2015;30:2086-95.
https://doi.org/10.1002/jbmr.2559.



3. Shin YH, Hong WK, Kim J, et al. Osteoporosis care after distal radius fracture reduces subsequent hip or spine fractures: a 4-year longitudinal study. Osteoporos Int 2020;31:1471-6.
https://doi.org/10.1007/s00198-020-05410-3.


4. Lewiecki EM. Osteoporosis: treat-to-target. Curr Osteoporos Rep 2017;15:103-9.
https://doi.org/10.1007/s11914-017-0350-7.


5. Lewiecki EM, Kendler DL, Davison KS, et al. Western osteoporosis alliance clinical practice series: treat-to-target for osteoporosis. Am J Med 2019;132:e771-e7.
https://doi.org/10.1016/j.amjmed.2019.04.044.


6. Vranken L, Wyers CE, van Rietbergen B, et al. The association between prevalent vertebral fractures and bone quality of the distal radius and distal tibia as measured with HR-pQCT in postmenopausal women with a recent non-vertebral fracture at the Fracture Liaison Service. Osteoporos Int 2019;30:1789-97.
https://doi.org/10.1007/s00198-019-05081-9.



7. Johansson L, Sundh D, Magnusson P, et al. Grade 1 vertebral fractures identified by densitometric lateral spine imaging predict incident major osteoporotic fracture independently of clinical risk factors and bone mineral density in older women. J Bone Miner Res 2020;35:1942-51.
https://doi.org/10.1002/jbmr.4108.


8. Schousboe JT, Langsetmo L, Szulc P, et al. Joint associations of prevalent radiographic vertebral fracture and abdominal aortic calcification with incident hip, major osteoporotic, and clinical vertebral fractures. J Bone Miner Res 2021;36:892-900.
https://doi.org/10.1002/jbmr.4257.



9. Kaneko A, Naito K, Nagura N, et al. Characteristics of sagittal spine alignment in female patients with distal radius fractures due to fall. Heliyon 2020;6:e04756.
https://doi.org/10.1016/j.heliyon.2020.e04756.



10. Lin T, Lu J, Zhang Y, et al. Does spinal sagittal imbalance lead to future vertebral compression fractures in osteoporosis patients? Spine J 2021;21:1362-75.
https://doi.org/10.1016/j.spinee.2021.03.014.


11. Heidsieck C, Gajny L, Travert C, et al. Effect of postural alignment alteration with age on vertebral strength. Osteoporos Int 2022;33:443-51.
https://doi.org/10.1007/s00198-021-06093-0.


12. Asahi R, Nakamura Y, Kanai M, et al. Association with sagittal alignment and osteoporosis-related fractures in outpatient women with osteoporosis. Osteoporos Int 2022;33:1275-84.
https://doi.org/10.1007/s00198-021-06282-x.


13. Genant HK, Wu CY, van Kuijk C, et al. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993;8:1137-48.
https://doi.org/10.1002/jbmr.5650080915.


14. Mallmin H, Ljunghall S. Distal radius fracture is an early sign of general osteoporosis: bone mass measurements in a population-based study. Osteoporos Int 1994;4:357-61.
https://doi.org/10.1007/bf01622198.


15. Fitzpatrick SK, Casemyr NE, Zurakowski D, et al. The effect of osteoporosis on outcomes of operatively treated distal radius fractures. J Hand Surg Am 2012;37:2027-34.
https://doi.org/10.1016/j.jhsa.2012.06.025.


16. Dontas IA, Yiannakopoulos CK. Risk factors and prevention of osteoporosis-related fractures. J Musculoskelet Neuronal Interact 2007;7:268-72.

17. Koo OT, Tan DM, Chong AK. Distal radius fractures: an epidemiological review. Orthop Surg 2013;5:209-13.
https://doi.org/10.1111/os.12045.



18. Kim J, Hwang JY, Oh JK, et al. The association between whole body sagittal balance and risk of falls among elderly patients seeking treatment for back pain. Bone Joint Res 2017;6:337-44.
https://doi.org/10.1302/2046-3758.65.Bjr-2016-0271.R2.



19. Giddins G, Giddins H. Wrist and hand postures when falling and description of the upper limb falling reflex. Injury 2021;52:869-76.
https://doi.org/10.1016/j.injury.2020.11.056.


20. Hsiao ET, Robinovitch SN. Common protective movements govern unexpected falls from standing height. J Biomech 1998;31:1-9.
https://doi.org/10.1016/s0021-9290(97)00114-0.


21. Kelsey JL, Prill MM, Keegan TH, et al. Reducing the risk for distal forearm fracture: preserve bone mass, slow down, and don’t fall! Osteoporos Int 2005;16:681-90.
https://doi.org/10.1007/s00198-004-1745-8.


22. Zhang YL, Shi LT, Tang PF, et al. Correlation analysis of osteoporotic vertebral compression fractures and spinal sagittal imbalance. Orthopade 2017;46:249-55.
https://doi.org/10.1007/s00132-016-3359-1.


23. Langella F, Balestrino A, Damilano M, et al. The aging spine: the effect of vertebral fragility fracture on sagittal alignment. Arch Osteoporos 2021;16:109.
https://doi.org/10.1007/s11657-021-00975-w.


24. Yagi M, Hosogane N, Watanabe K, et al. The paravertebral muscle and psoas for the maintenance of global spinal alignment in patient with degenerative lumbar scoliosis. Spine J 2016;16:451-8.
https://doi.org/10.1016/j.spinee.2015.07.001.


25. Park JS, Park YS, Kim J, et al. Sarcopenia and fatty degeneration of paraspinal muscle associated with increased sagittal vertical axis in the elderly: a cross-sectional study in 71 female patients. Eur Spine J 2020;29:1353-61.
https://doi.org/10.1007/s00586-020-06416-5.


26. Shah GM, Gong HS, Chae YJ, et al. Evaluation and management of osteoporosis and sarcopenia in patients with distal radius fractures. Clin Orthop Surg 2020;12:9-21.
https://doi.org/10.4055/cios.2020.12.1.9.



27. Yurube T, Ito M, Takeoka T, et al. Possible improvement of the sagittal spinopelvic alignment and balance through “Locomotion Training” exercises in patients with “Locomotive Syndrome”: a literature review. Adv Orthop 2019;2019:6496901.
https://doi.org/10.1155/2019/6496901.



28. Genant HK, Li J, Wu CY, et al. Vertebral fractures in osteoporosis: a new method for clinical assessment. J Clin Densitom 2000;3:281-90.
https://doi.org/10.1385/jcd:3:3:281.


29. Hofbauer LC, Hamann C, Ebeling PR. Approach to the patient with secondary osteoporosis. Eur J Endocrinol 2010;162:1009-20.
https://doi.org/10.1530/eje-10-0015.


30. Lems WF, Paccou J, Zhang J, et al. Vertebral fracture: epidemiology, impact and use of DXA vertebral fracture assessment in fracture liaison services. Osteoporos Int 2021;32:399-411.
https://doi.org/10.1007/s00198-020-05804-3.



31. Margulies JY, Payzer A, Nyska M, et al. The relationship between degenerative changes and osteoporosis in the lumbar spine. Clin Orthop Relat Res 1996;324:145-52.
https://doi.org/10.1097/00003086-199603000-00017.


32. De Laet C, Kanis JA, Odén A, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int 2005;16:1330-8.
https://doi.org/10.1007/s00198-005-1863-y.


33. Compston JE, Watts NB, Chapurlat R, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med 2011;124:1043-50.
https://doi.org/10.1016/j.amjmed.2011.06.013.



34. Court-Brown CM, Duckworth AD, Ralston S, et al. The relationship between obesity and fractures. Injury 2019;50:1423-8.
https://doi.org/10.1016/j.injury.2019.06.016.


35. Xu W, Ni C, Yu R, et al. Risk factors for distal radius fracture in postmenopausal women. Orthopade 2017;46:447-50.
https://doi.org/10.1007/s00132-017-3403-9.


36. Acosta-Olivo C, Gonzalez-Saldivar JC, Villarreal-Villarreal G, et al. Correlation between obesity and severity of distal radius fractures. Orthop Traumatol Surg Res 2017;103:199-202.
https://doi.org/10.1016/j.otsr.2016.12.007.


- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,305 View
- 24 Download
- ORCID iDs
-
Jeong Hyun Lee

https://orcid.org/0000-0002-6860-3444Hansang Lee

https://orcid.org/0000-0001-9332-6568Hyun Sik Gong

https://orcid.org/0000-0003-4028-1559 - Related articles




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print