|
 |
| jbm > Volume 30(4); 2023 > Article |
|
Abstract
Background
Methods
Results
DECLARATIONS
Ethics approval and consent to participate
The study protocol conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board of Chung-Ang University Hospital, Gyeongsang National University Hospital, Yonsei University Health System, Kyung Hee University Healthcare System, Pusan National University Hospital, Seoul National University Bundang Hospital, and Inha University Hospital. Informed consent was obtained from participants.
Fig. 1
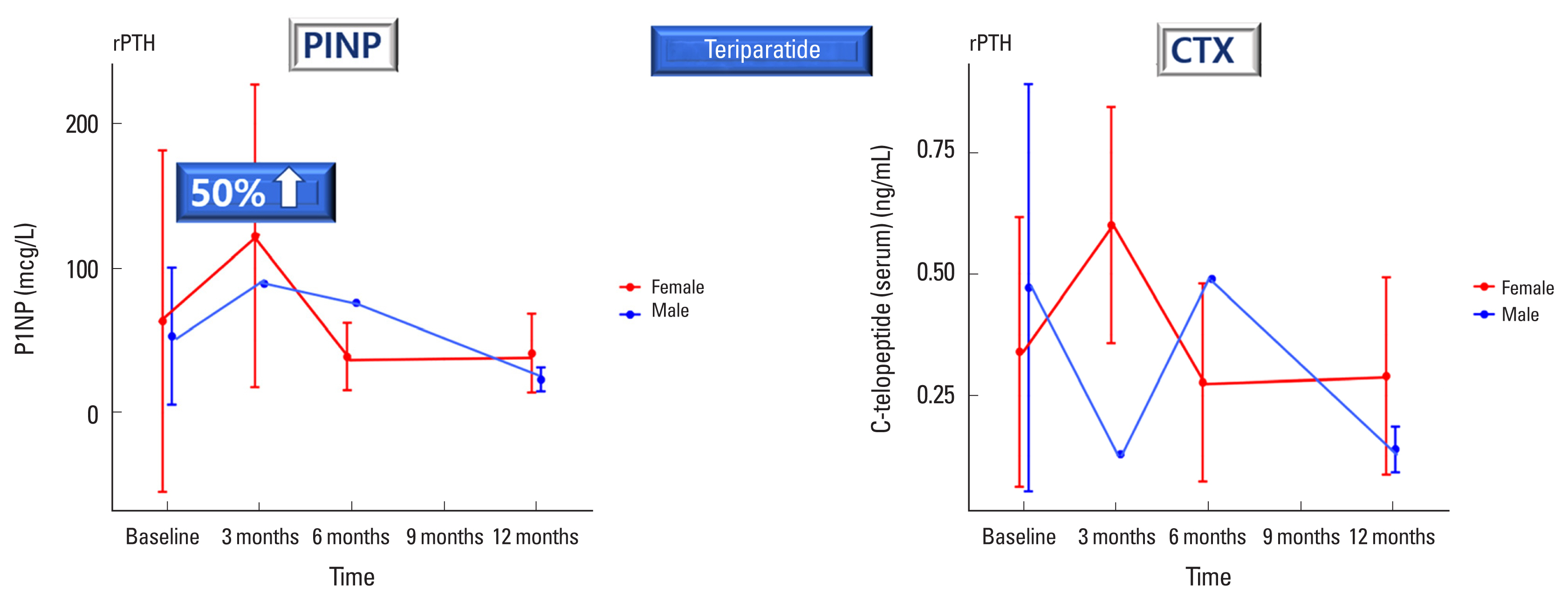
Fig. 2
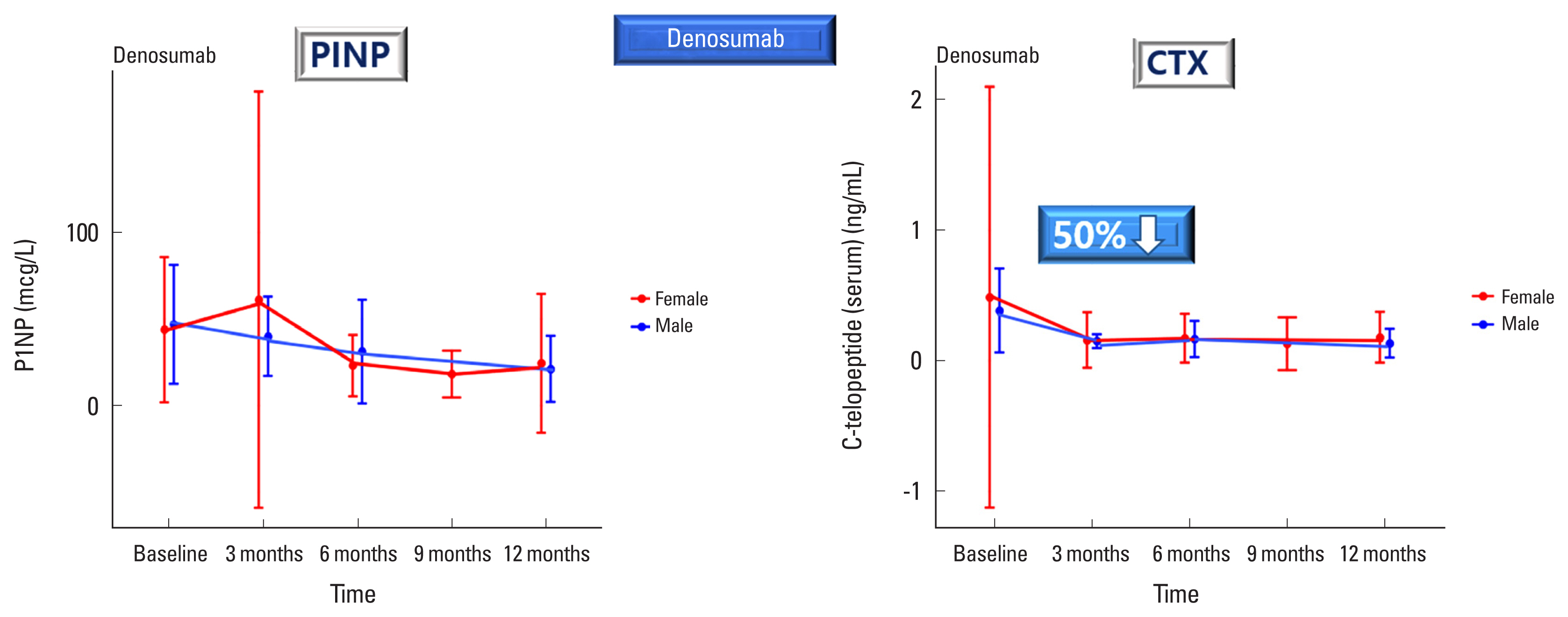
Fig. 3

Table 1
Table 2
| Variables | N | Mean±SD | Median | Q25% | Q75% |
|---|---|---|---|---|---|
| P1NP (mcg/L) | |||||
| Female | 410 | 45.024±48.099 | 34.935 | 21.405 | 53.263 |
| Male | 43 | 54.971±98.508 | 28.98 | 21.535 | 47.85 |
|
|
|||||
| C-telopeptide (serum) (ng/mL) | |||||
| Female | 291 | 0.339±0.843 | 0.232 | 0.141 | 0.382 |
| Male | 32 | 0.405±0.499 | 0.262 | 0.195 | 0.466 |
|
|
|||||
| Osteocalcin (serum) (ng/mL) | |||||
| Female | 26 | 13.36±53.408 | 0 | 0 | 5.865 |
| Male | 1 | 13.6a) | 13.6 | 13.6 | 13.6 |
Table 3
Table 4
Table 5
| Variables | Osteoporosisa) (N=291) | Normalb) (N=472) | Total (N=763) |
|---|---|---|---|
| P1NP (mcg/L) |
65.768±94.373 40.400 (25.300-70.800) |
48.026±49.364 40.050 (25.500-57.050) |
54.793±70.499 40.300 (25.450-60.550) |
| C-telopeptide (ng/mL) |
0.528±1.206 0.358 (0.173-0.613) |
0.361±0.673 0.276 (0.160-0.463) |
0.425±0.918 0.303 (0.164-0.505) |
REFERENCES
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,787 View
- 66 Download
- ORCID iDs
-
Jun-Il Yoo

https://orcid.org/0000-0002-3575-4123So Young Park

https://orcid.org/0000-0002-4820-9415Deog-Yoon Kim

https://orcid.org/0000-0003-4054-0231Jeonghoon Ha

https://orcid.org/0000-0001-9219-7135Yumie Rhee

https://orcid.org/0000-0003-4227-5638Namki Hong

https://orcid.org/0000-0002-8246-1956Jung-Taek Kim

https://orcid.org/0000-0003-4243-5793Hyon-Seung Yi

https://orcid.org/0000-0002-3767-1954Bu Kyung Kim

https://orcid.org/0000-0001-7845-4377Young-Kyun Lee

https://orcid.org/0000-0001-6564-4294Yong-Chan Ha

https://orcid.org/0000-0002-6249-0581Yun Kyung Jeon

https://orcid.org/0000-0002-4319-5181Ha-Young Kim

https://orcid.org/0000-0002-0651-2213Seong Hee Ahn

https://orcid.org/0000-0003-2558-2118Seongbin Hong

https://orcid.org/0000-0002-8189-395XSang-Yeob Lee

https://orcid.org/0000-0001-8024-1135 - Related articles
-
Position Statement on the Use of Bone Turnover Markers for Osteoporosis Treatment2019 November;26(4)
Clinical Application of Bone Turnover Markers in Osteoporosis in Korea2019 February;26(1)





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print