Effects of Bazedoxifene/Vitamin D Combination Therapy on Serum Vitamin D Levels and Bone Turnover Markers in Postmenopausal Women with Osteopenia: A Randomized Controlled Trial
Article information
Abstract
Background
This study aimed to evaluate the effectiveness of bazedoxifene/vitamin D combination therapy in preventing osteoporosis in postmenopausal women with osteopenia.
Methods
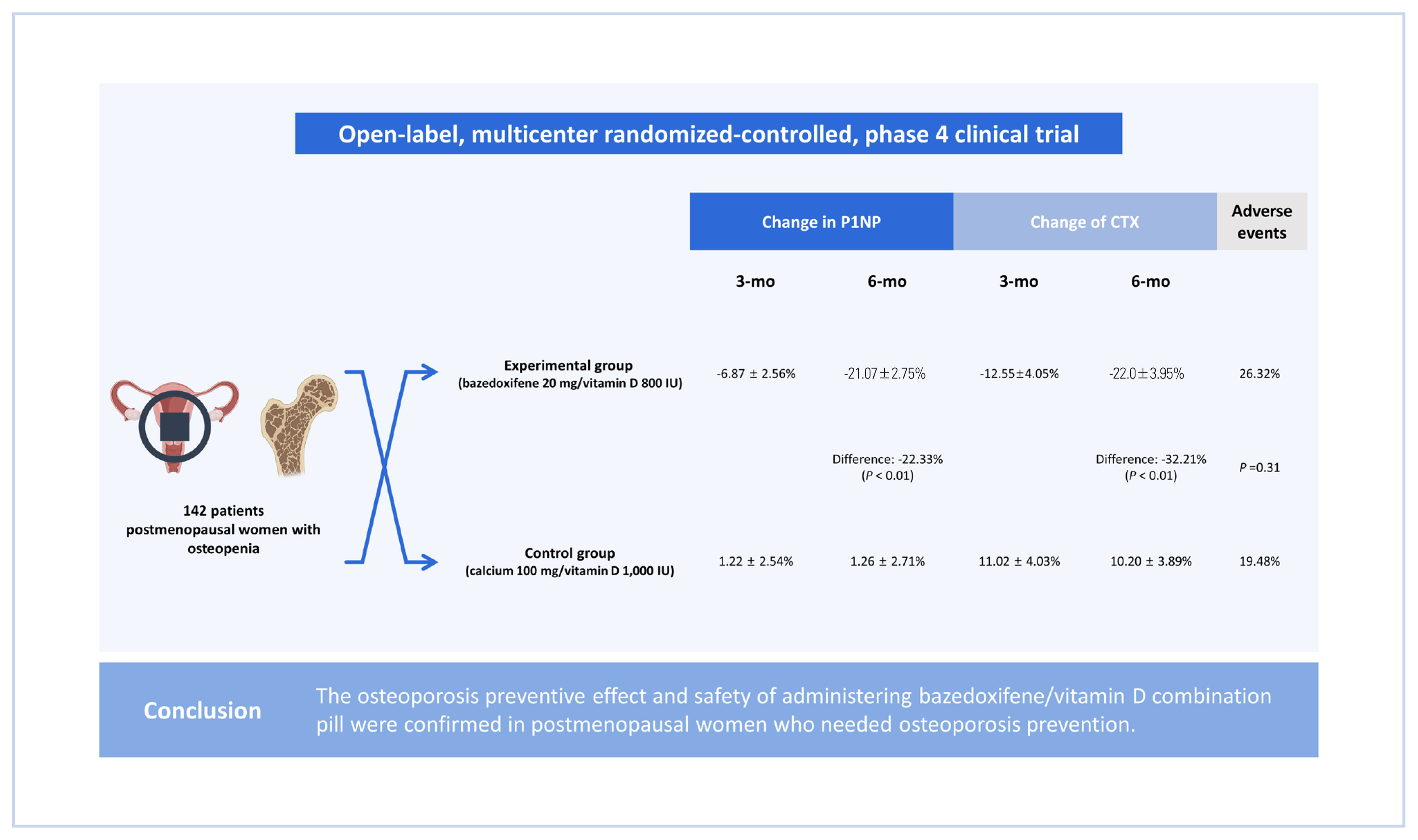
This was an open-label, multicenter randomized-controlled, phase 4 clinical trial. Women between ages of 55 and 70 years in 9 medical tertiary centers in Korea were enrolled and assigned into 2 groups: an experiment group and a control group. The experimental group received bazedoxifene 20 mg/vitamin D 800 IU tablets for 6 months, and the control group received calcium 100 mg/vitamin D 1,000 IU tablets for 6 months.
Results
A total of 142 patients (70 in the experimental group and 72 in the control group) were included. The least-square mean±standard error of change in propeptide of type I collagen after 3 months was −6.87±2.56% in the experimental group and 1.22±2.54% in the control group. After 6 months, it was −21.07±2.75% in the experimental group and 1.26±2.71% in the control group. The difference between the 2 groups was −22.33% (P<0.01). The change of C-terminal telopeptide was −12.55±4.05% in the experimental group and 11.02±4.03% in the control group after 3 months. It was −22.0±3.95% and 10.20±3.89, respectively, after 6 months. The difference between the 2 groups was −32.21% (P<0.01) after 6 months. There was no significant difference in adverse events between the 2 groups.
Conclusions
The osteoporosis preventive effect and safety of administering bazedoxifene/vitamin D combination pill were confirmed in postmenopausal women who needed osteoporosis prevention.
INTRODUCTION
Osteoporosis is defined as “a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue with a consequent increase in bone fragility and susceptibility to fracture”.[1] According to the Korean Society for Bone and Mineral Research (KSBMR), the prevalence of osteoporosis in women aged 50 years or older was 37.3% in South Korea.[2] The prevalence of osteoporosis is increasing worldwide.[3] The social burden caused by osteoporosis will continue to increase in Korea, rapidly entering a super-aged society.[4,5] Since it is difficult to treat osteoporosis after its occurrence, it is important to prevent osteoporosis from a pre-osteoporosis stage. Therefore, it is more important to manage and prevent bone metabolism in osteopenia patients, especially in postmenopausal osteopenia patients.[6]
Most osteoporosis drugs currently used are bone resorption inhibitors. Bisphosphonates and selective estrogen receptor modulators (SERMs) are representative drugs. Among them, SERMs can inhibit bone resorption in postmenopausal women and prevent a decrease in bone strength. Bazedoxifene is a SERM-type drug that can act as an estrogen receptor antagonist in the uterus and breast to inhibit the proliferation of endometrial and breast epithelial tissues. It can also act as an estrogen receptor agonist to regulate bone metabolism and lipid metabolism, thus maintaining bone density and lower lipid levels. The US Food and Drug Administration has approved bazedoxifene for the prevention and treatment of postmenopausal osteoporosis and European Medicines Agency has approved the use of bazedoxifene for women with postmenopausal osteoporosis at increased risk of fractures.
Vitamin D is an endogenous substance that plays an essential role in the regulation of bone and mineral metabolism. Insufficient levels of vitamin D can cause a decrease in the absorption of calcium and disrupt the process of bone remodeling. This can lead to reduced bone mineral density (BMD), weakened bones, and an increased likelihood of fractures.[7,8] As a result, several guidelines including that of the KSBMR recommend a vitamin D intake of 600 to 1,000 IU per day for adults to reduce the risk of fractures.[9–11]
Recently, a combination of bazedoxifene and vitamin D has been developed and used as a treatment for osteoporosis. The single-pill combination is expected to lead to better adherence. The importance of drug adherence in treating osteoporosis to achieve the goal of fracture prevention is well-recognized and emphasized in several prior studies.[12,13] There is a general agreement that an effective way to improve treatment adherence is treatment simplification, i.e., reducing the number of pills to be taken daily.[14,15] However, studies on whether this combination is effective in preventing osteoporosis are lacking.
Therefore, the objective of this study was to perform an exploratory comparative analysis to determine whether there were differences in the preventive effect on osteoporosis and safety between the group using bazedoxifene and vitamin D combination pill and the group using general treatment (calcium and vitamin D) in postmenopausal women who needed osteoporosis prevention.
METHODS
1. Study enrollment
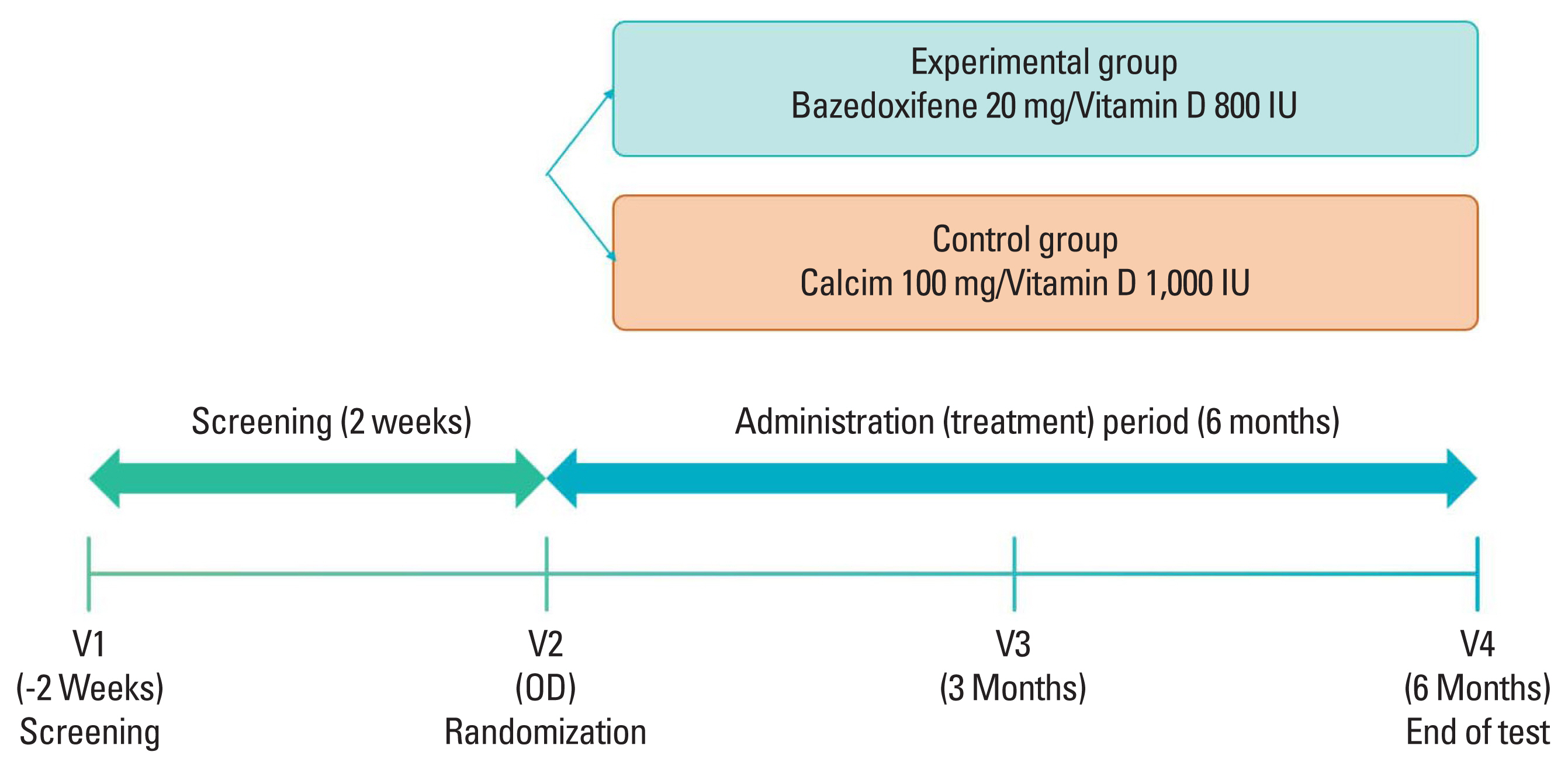
This was an open-label, randomized multicenter controlled phase 4 clinical trial to verify preventive effects of administering bazedoxifene/vitamin D combination pill for 6 months on osteoporosis in postmenopausal patients. Nine medical tertiary centers in Korea participated in this study. Bone density was measured for women aged 55 to 70 years. Participants were enrolled when the lowest T score was in the osteopenia range (between −2.5 and −1.0). The precise full enrollment principle and study timetable are described in Supplementary Table 1 and 2. We estimated that a sample size of 150 participants would provide the study with 80% power to detect a 5% difference in the means, assuming a pooled standard deviation (SD) of 10.3% and a 2-sided type I error of 0.05. We also accounted for a 10% loss to follow-up. When the subject gave written consent to participate in this clinical trial, a screening number was assigned and the subject’s screening test results were evaluated. Only subjects who met the selection criteria and did not meet the exclusion criteria were assigned a randomization number and clinical trial drug number (only for the experimental group) through the Interactive Web Response System. A central randomization method was applied by setting the ratio of the experimental group to the control group at 1:1 using the Proc Plan Procedure of SAS (Version 9.4; SAS institute, Cary, NC, USA). The experimental group received bazedoxifene 20 mg/vitamin D 800 IU tablets for 6 months and the control group received calcium 100 mg/vitamin D 1,000 IU tablets for 6 months. These tablets were used to prevent osteoporosis in a daily medical environment. At 6 months, scheduled examinations were conducted and the clinical trial was terminated (Fig. 1). Biochemical tests such as serum cross-linked C-terminal telopeptide (CTX; a bone resorption marker), serum propeptide of type I collagen (P1NP; a bone formation marker), serum 25-hydroxy-vitamin were measured through blood sampling in the same morning time zone as possible in an empty state of at least 8 hr. During the entire test period, subjects were prohibited from taking drugs that could affect bone or calcium metabolism other than the given drugs. Contraindicated drugs are described in Supplementary Table 3. This study complied with the Declaration of Helsinki and was performed according to ethics committee approval.
2. Study endpoints
Primary endpoints of this study were changes in P1NP and CTX at 3 and 6 months after administration compared to baseline. Secondary efficacy endpoints were quality of life evaluation (EuroQol-5-dimensions 5-level [EQ-5D-5L]) scores and treatment satisfaction (5-point Likert scale) at 3 months and 6 months after administration. EQ-5D, a generic instrument for describing and valuing health, is based on a descriptive system that defines health in 5-dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The latest version is referred to as the EQ-5D-5L.[16] An EQ-5D-5L with Korean preference weights based on values obtained for the Korean population was used as a protocol.[17] The questionnaire for determining treatment satisfaction was evaluated on a 5-point Likert scale (1=‘Not at all’, 2=‘Not so’, 3=‘Average’, 4=‘Agree’, and 5=‘Very agree’).
For adverse events, the incidence rate and the number of occurrences of adverse events were analyzed for 153 subjects (safety analysis population) who had undergone randomization and taken at least one dose of bazedoxifene combination pill or control drug. ‘Adverse Event’ refers to undesirable and unintended signs (signs, e.g., abnormal laboratory tests), symptoms, or diseases that occur during the administration and use of pharmaceuticals. It does not necessarily have to have a causal relationship. ‘Drug-related adverse events’ refers to a harmful and unintended reaction caused by normal administration and use of a drug from which a causal relationship with the drug cannot be excluded.
A person in charge of the clinical trial checked medication compliance based on the number of untaken drugs collected or the number of medicines brought by the subject. Medication compliance was calculated as follows:
Medication (treatment) compliance (%)=Number of medications taken/Number of medications to be taken×100
3. Statistical analysis
Efficacy analyses were performed in the full analysis set (all participants who had at least one valid efficacy evaluation) according to the intention-to-treat principle. To compare change rates of clinical markers after 6 months of administration compared to baseline, descriptive statistics (mean, SD, median, minimum, maximum) for each group were used and an analysis of covariance (ANCOVA) using baseline value as a covariate was performed. Within-group changes after treatment compared to baseline were tested by paired t-test or Wilcoxon’s signed rank test. Regarding the EQ-5D-5L score, the difference between groups was compared using a 2-sample t-test or Wilcoxon’s rank sum test. Treatment satisfaction evaluation after 3 months and 6 months of administration (5-point Likert scale) was performed for each group. Differences between groups were compared using χ2 test or Fisher’s exact test. For all estimated between-group differences, 2-sided 95% confidence intervals (CIs) were calculated.
RESULTS
1. Baseline characteristics
Of the 173 participants who were screened, a total of 157 underwent randomization. Four participants were excluded from the safety set because the trial drug was not administered properly. Additionally, 6 participants in the experimental group and 5 in the control group were excluded from the full analysis population because they did not undergo primary efficacy evaluation. In total, 70 participants were assigned to the experimental group and 72 were assigned to the control group in the full analysis set. Nine participants were dropped from each group due to inadequate inclusion/exclusion criteria, contraindicated drug use, non-compliance with medication, withdrawal of consent, and serious protocol violations. As a result, treatment was completed for 61 participants in the experimental group and 66 in the control group at 6 months (Fig. 2).
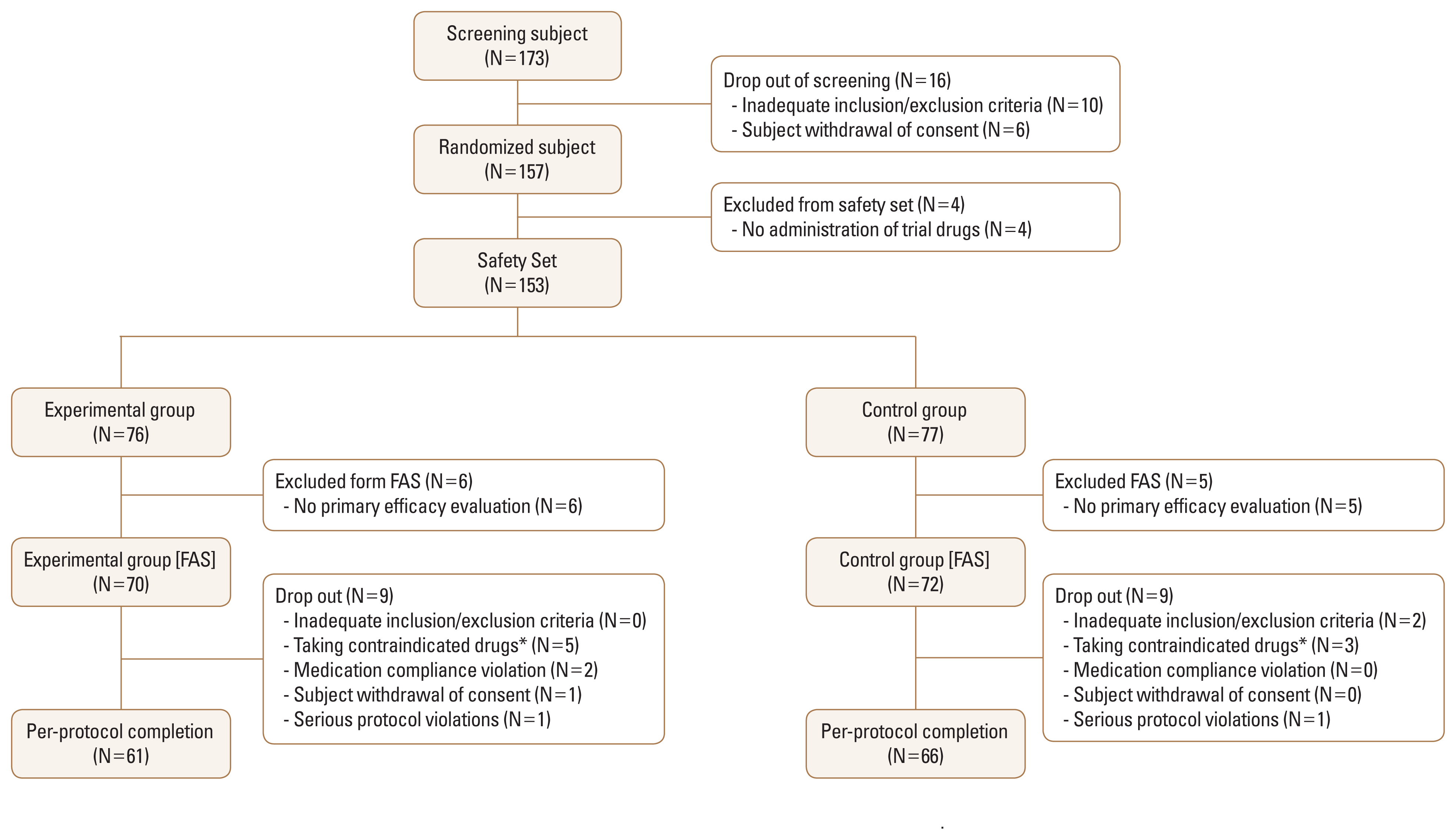
Flowchart showing study population enrollment. FAS; full analysis set. *See Supplementary Table 3 for more details.
The baseline characteristics of study participants are described in Table 1. Demographic information like age, menopausal age, smoking, drinking, caffeine intake, and osteoporosis risk factors were identified. There were no statistically significant differences in baseline characteristics between the experimental group and the control group except for the level of CTX. The mean age was 62.9±3.9 years and the mean body mass index was 23.9±3.2 kg/m2. The mean vitamin D level was 32.3±12.7 ng/mL. Additional data are described in Supplementary Table 4.
2. Results of bone turnover markers (BTMs)
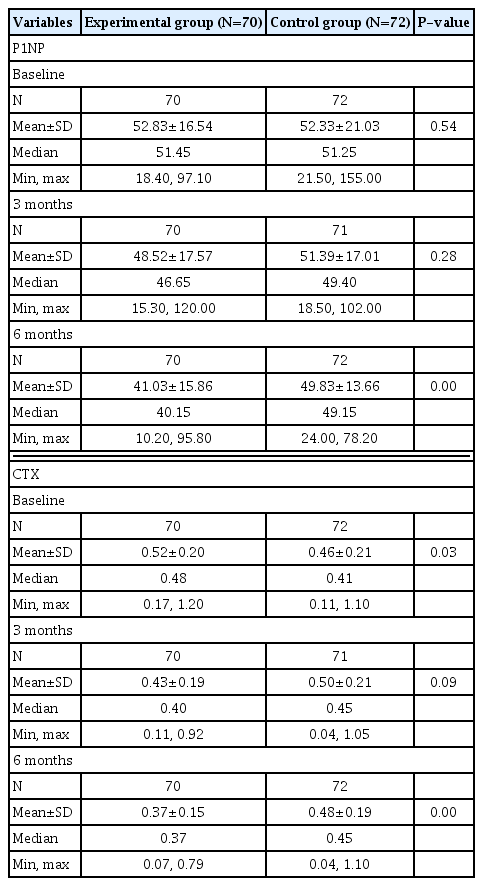
Both P1NP and CTX levels decreased in the group administered with bazedoxifene and vitamin D combination pill (Table 2). The least-square mean±standard error (LS mean±SE) of the change rate of P1NP after 3 months of administration of the test drug compared to baseline was −6.87±2.56% in the experimental group (N=70) and 1.22± 2.54% in the control group (N=72). After 6 months, it was −21.07±2.75% in the experimental group and 1.26±2.71% in the control group. The difference between the 2 groups was −22.33% (95% CI, −29.96, −14.71), which was statistically significant (P<0.01). The change rate of CTX after administration of the investigational drug compared to baseline was −12.55±4.05 % in the experimental group and 11.02± 4.03 % in the control group at 3 months. It was −22.01±3.95% in the experimental group and 10.20±3.89% in the control group at 6 months. The difference between the 2 groups was −32.21% (95% CI, −43.23, −21.20) at 6 months, which was statistically significant (P<0.01) (Fig. 3A, 3B).
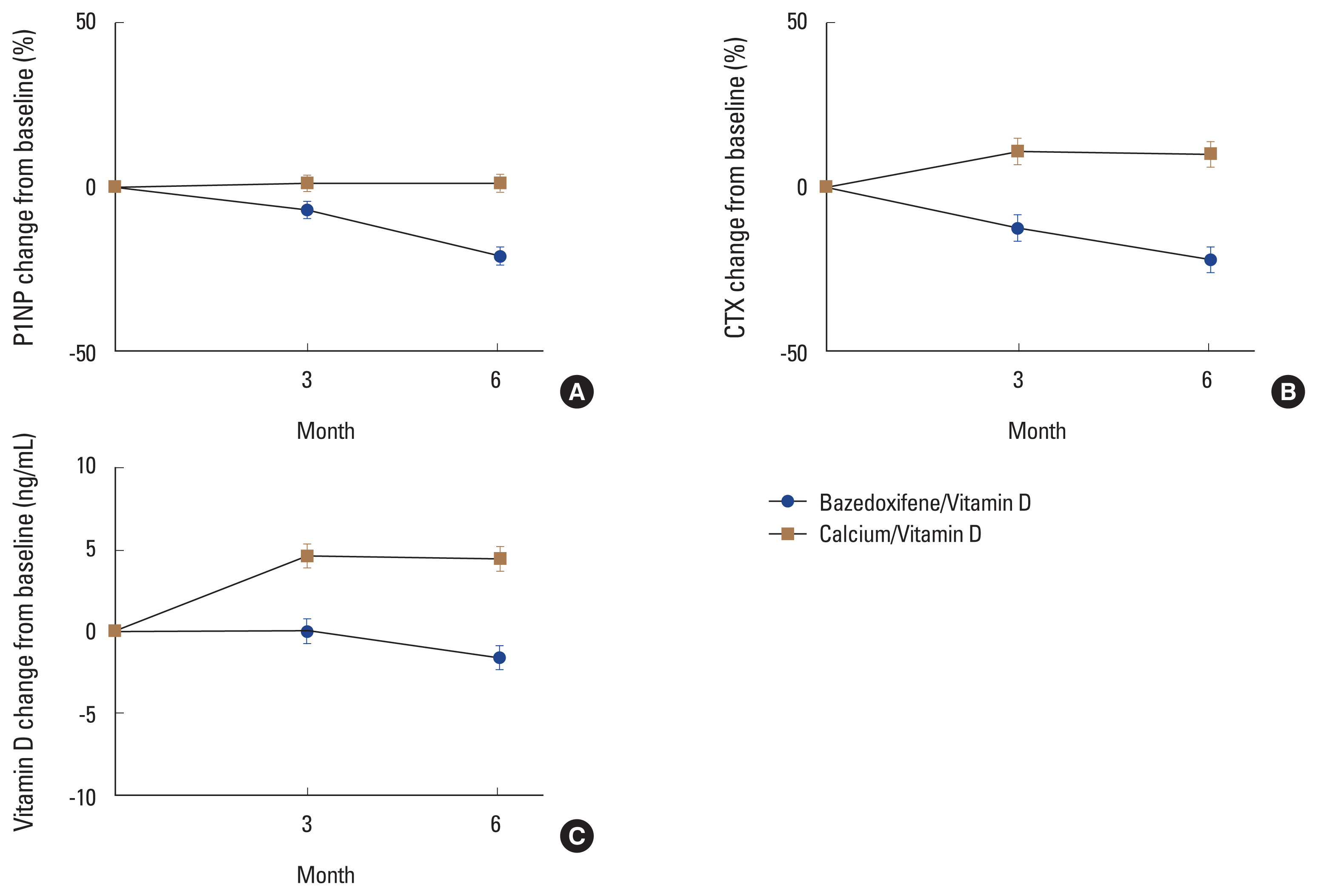
Changes of bone turnover markers and vitamin D in the bazedoxifene/vitamin D group and the calcium/vitamin D group. Error bars represent the standard deviation. (A) Propeptide of type I collagen (P1NP). (B) C-terminal telopeptide (CTX). (C) Vitamin D.
The LS mean±SE change rate of vitamin D at 3 months compared to baseline was 0.03±0.75 ng/mL in the experimental group and 4.64±0.74 ng/mL in the control group. At 6 months, it was −1.59±0.75 ng/mL in the experimental group and 4.50±0.74 ng/mL in the control group. The difference between the 2 groups was −6.09 ng/mL (95% CI, −8.16, −4.02 ng/mL) at 6 months, which was statistically significant (P<0.01) (Fig. 3C).
3. Results in quality of life evaluation and satisfaction rate
The descriptive system index score (mean±SD) for quality of life evaluation (EQ-5D-5L) was 0.86±0.09 points in the experimental group and 0.87±0.05 points in the control group at 3 months. It was 0.87±0.07 points in the experimental group and 0.86±0.05 points in the control group at 6 months. It was not significantly different between the 2 groups at 3 months or 6 months (3 months, P=0.67; 6 months, P=0.38). The EQ-VAS score (mean±SD) among EQ-5D-5L was 79.34±15.80 points in the experimental group and 81.76±13.50 points in the control group at 3 months. It was 81.37±11.41 points in the experimental group and 80.07±13.40 points in the control group at 6 months. These scores were not significantly different between the 2 groups at 3 months or 6 months (3 months, P=0.25; 6 months, P=0.82). As a result of treatment satisfaction evaluation (5-point Likert scale), there was no statistically significant difference between the 2 groups (3 months, P=0.57; 6 months, P=0.54) (Supplementary Table 5).
4. Safety results
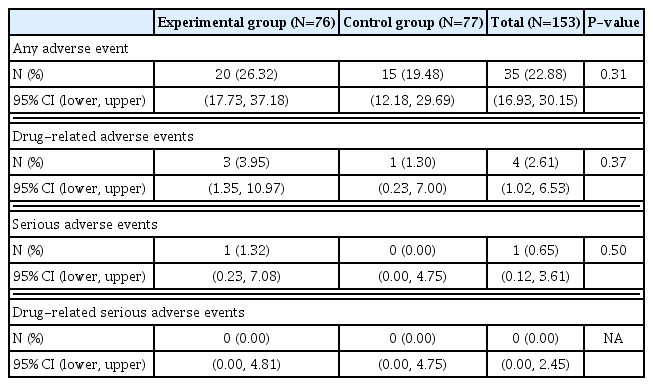
A total of 35 adverse events occurred out of 153 subjects, including 20 (26.32%) of 76 subjects in the experimental group and 15 (19.48%) of 77 subjects in the control group. There was no statistically significant difference in the incidence of adverse reactions (P=0.31) between the 2 groups (Table 3). A total of 4 drug-related adverse events occurred in 4 subjects, including 3 (3.95%) cases in the experimental group and 1 (1.30%) case in the control group. Adverse drug reactions included ‘mild headache’, ‘paraesthesia’, and ‘mild urticaria’ in the experimental group and ‘moderate gastrointestinal disorder’ in the control group. Adverse reactions that led to dropout were 2 cases (headache and urticaria) in the experimental group and one (gastrointestinal disorder) in the control group. All patients with drug-related adverse events recovered after discontinuing or with general medical care. No fatal adverse event was reported. One severe adverse event (ankle fracture) occurred in the experimental group. It was ‘unlikely’ to be caused by the drug. The fracture recovered with maintenance of the experimental drug. More detailed information for adverse events are described in Supplementary Table 6.
5. Medication compliance
The mean medication compliance (±SD) was 95.87±7.38% in the full analysis set. The experimental group (N=70) was 95.40±8.82% and the control group (N=72) was 96.32 ±5.68%. Both groups showed an average of 95% or higher medication compliance. There was no statistically significant difference in mean medication compliance between the 2 groups (P=0.63).
DISCUSSION
This 6-month multicenter clinical trial was conducted to exploratory confirm the preventive effect of administering bazedoxifene 20 mg/vitamin D 800 IU on osteoporosis in postmenopausal women. Based on efficacy evaluation results, bone formation marker (P1NP) and bone resorption marker (CTX) at 6 months after administration were significantly decreased in the experimental group compared to those at baseline. Their reduction rates were greater in the experimental group than in the control group.
In this study, bazedoxifene/vitamin D was associated with a median percent reduction from baseline of −6.87% at 3 months and −21.07% at 6 months in serum P1NP. This result at 6 months was similar to an earlier study showing a −19.2% decrease in osteocalcin after 6 months of treatment with bazedoxifene.[18] Likewise, a phase 2 placebo-controlled trial conducted with western postmenopausal women has documented a −6.08% decrease in osteocalcin after 3 months of treatment.[19] Moreover, our study reported a decrease of −22.0% in CTX, a bone resorption marker. This coincided well with previous studies showing a −30.6% median percent reduction from baseline in serum CTX after 6 months and a −24% decrease after 24 months.[18,20]
Zoledronic acid, the most potent bisphosphonate, is known to decrease bone resorption markers by about 50% to 70% after 6 months of treatment, while denosumab reduces bone resorption by 70% to 80% after the same duration of treatment.[21–23] On the other hand, SERMs decrease bone resorption by approximately 30%.[24] Our results with bazedoxifene/vitamin D were in accordance with those of previous data.
Several studies including meta-analysis and population-based studies have demonstrated the clinical usefulness of BTM concentration in osteoporosis in association with fracture risk reduction.[25–30] Studies suggested that the BTM can offer insights into fracture risk regardless of BMD. Therefore, we postulate that changes in BTMs in this study could reflect a reduced risk of fracture risk. Likewise, Bruyère and colleagues’ study [28] on postmenopausal women with osteoporosis who received bazedoxifene 20 mg has reported a relationship between changes in BTM (CTX and osteocalcin) and fracture risk reduction. Therefore, the use of bazedoxifene/vitamin D combination pill might be beneficial for the prevention of future fractures.
The quality of life evaluation and satisfaction rate of the bazedoxifene/vitamin D combination pill did not differ from those of the control group. Furthermore, the safety profile of bazedoxifene/vitamin D combination pill was consistent with that observed with bazedoxifene-only pills in general. No new safety concern was identified. In previous studies, hot flushes and leg cramps were more frequent in the group administered with bazedoxifene-only pills than in the placebo group.[31,32] Leg cramps and hot flushes are known side effects of SERM.[33,34] However, our study participants did not report leg cramps or hot flushes. Recently, a link between vasomotor symptoms and vitamin D levels has been getting attention.[35] Vitamin D in the experimental drug might have alleviated drug-class-related adverse events. Further follow-up is needed to clarify this postulation.
This study has several limitations. First, since Vitamin D only pill is not well used in Korea, the control group was prescribed a calcium 100 mg/vitamin D 1,000 IU combination pill whereas the experimental group had bazedoxifene/vitamin D 800 IU combination pill. Although the control group had an extra dose of calcium compared to the experimental group, the 100 mg dose of calcium is relatively small considering that the recommended daily dose of calcium is 1,000 mg by the KSBMR.[36] We think this 100 mg calcium difference is small enough without affecting outcomes of this study. Second, there is a dose difference of 200 IU in vitamin D between the 2 groups. However, despite a lower level of vitamin D, the group administered with bazedoxifene demonstrated a more significant BTM reduction. The addition of vitamin D is believed to have a beneficial effect on bone health, although it does not affect the results of the BTM. Several studies have shown that vitamin D supplementation typically does not impact BTM levels.[37,38] Therefore, it is unlikely to have impacted the validity of its conclusion. Third, this study evaluated BTM rather than BMD. Since the Korea National Health Insurance system only approves annual BMD measurement, endpoint of this 6-month study was BTM instead of BMD. As mentioned, although BTM indicates bone health or fracture risk, further studies with the evaluation of BMD for preventing osteoporosis are in need.[25–28] Lastly, this study was conducted only in South Korea. Results might not be applicable to other populations.
In summary, this study confirms the osteoporosis preventive effect and safety of administering the bazedoxifene/vitamin D complex in postmenopausal women with osteopenia.
Supplementary Information
Notes
Funding
This study was supported by Alvogen Korea.
Ethics approval and consent to participate
Not applicable.
Conflict of interest
No potential conflict of interest relevant to this article was reported.