Moderate-Intensity Exercise Preserves Bone Mineral Density and Improves Femoral Trabecular Bone Microarchitecture in Middle-Aged Mice
Article information
Abstract
Background
Aging leads to significant bone loss and elevated osteoporosis risk. Exercise slows age-related bone loss; however, the effects of various moderate-intensity exercise training volumes on bone metabolism remain unclear. This study aimed to determine the degree to which different volumes of moderate-intensity aerobic exercise training influence bone mineral density (BMD), bone mineral content (BMC), femoral trabecular bone microarchitecture, and cortical bone in middle-aged mice.
Methods
Twenty middle-aged male C57BL/6 mice were randomly assigned 8 weeks of either (1) non-exercise (CON); (2) moderate-intensity with high-volume exercise (EX_MHV); or (3) moderate-intensity with low-volume exercise (EX_MLV) (N=6–7, respectively). Femoral BMD and BMC were evaluated using dual energy X-ray absorptiometry, and trabecular and cortical bone were measured using micro-computed tomography.
Results
Femoral BMD in EX_MHV but not EX_MLV was significantly higher (P<0.05) than in CON. The distal femoral fractional trabecular bone volume/tissue volume (BV/TV, %) was significantly higher (P<0.05) in both EX_MHV and EX_MLV than in CON mice. Increased BV/TV was induced by significantly increased trabecular thickness (mm) and tended to be higher (P<0.10) in BV (mm3) and lower in trabecular separation (mm) in EX_MHV and EX_MLV than in CON. The femoral mid-diaphysis cortical bone was stronger in EX_MLV than EX_MHV.
Conclusions
Long-term moderate-intensity aerobic exercise with low to high volumes can be thought to have a positive effect on hindlimb BMD and attenuate age-associated trabecular bone loss in the femur. Moderate-intensity aerobic exercise may be an effective and applicable exercise regimen to prevent age-related loss of BMD and BV.
INTRODUCTION
Age-related alterations occur in the skeletal system associated with bone loss and increased fracture risks, leading to a myriad of pathological developments such as bone diseases like osteopenia and osteoporosis.[1] Osteoporosis can be conceived as a disorganized bone remodeling process where bone-forming cell, osteoblast, activity and bone resorption cell, osteoclast, activity were unbalanced, thereby the level of bone formation is smaller than that of bone resorption.[2] Osteoporosis causes chronic pain and reduced quality of life when it is in more severe expressions,[3] leading to increased healthcare-related funds and an important health issue [4] and increased mortality.[5,6] Even though the presence of known unfavorable clinical and experimental findings,[7–9] treatment options for osteoporosis in the elderly are available, such as a well-known bone anabolic agent treatment for osteoporosis, intermittent parathyroid hormone (iPTH),[10] and osteoclast activity inhibitor, denosumab.[9] Despite the fact that pharmaceutical methods are beneficial, non-pharmacochemical approaches, including nutrition and exercise, have been highlighted to prevent age-associated bone loss and osteoporosis.
Maintenance of a healthy lifestyle including habitual exercise with loading is a renowned non-pharmaceutical intervention that effectively improves bone metabolism by stimulating bone adaptation to mechanical forces.[11,12] The mechanical force induced by weight-bearing exercise (i.e., jumping) promotes osteocyte activity and mechano-sensor cells in bone tissue, stimulating bone remodeling via osteoblast and osteoclast differentiation and activity.[13] In addition, load-induced mechanical force generation during exercise inhibits osteoclastogenesis by stimulating osteoprotegerin expressions.[14] Indirectly to bone tissue, exercise also enhances bone vascular function, blood flow, and bone-associated skeletal nerve fiber density, which also alter the osteoblasts and osteoclasts differentiation via nitric oxide (NO)-mediated pathway [15,16] and via exercise-induced differential expression of microRNA.[17] Indeed, there have been several investigations reporting the beneficial effects of exercise on bone mineral density (BMD) and other bone properties.[18–20] However, to date, a fundamental understanding of how to exercise volumes within the same intensity modulates bone mineralization and bone volume (BV) as well as attenuate age-associated bone loss is lacking and is the focus of the current investigation.
To achieve this aim, we tested the hypothesis that a higher volume of long-term moderate-intensity aerobic exercise would result in greater increases in femoral BMD, bone mineral content (BMC), trabecular bone microarchitecture, and cortical bone strength parameters compared to lower volume moderate-intensity exercise and no exercise. To test this hypothesis, middle-aged mice (14-months old) were randomly assigned to 8-weeks of either (1) non-exercise (CON; N=6); (2) moderate-intensity with high-volume exercise (EX_MHV; N=7); (3) moderate-intensity with low-volume exercise (EX_MLV; N=7).
METHODS
1. Animals
The animal experimental protocol was approved by Institutional Animal Care and Use Committee (IACUC) of the Sungkyunkwan University School of Medicine (Approval no. SKKUIACUC 18-5-24-3) and followed the AAALAC International Guidelines for animal experiments. A total of 20 male C57BL/6 mice aged 14 months were purchased from Daehan Biolink Co. Ltd. (Daejeon, Korea). All mice were housed in standard cages at a pathogen-free animal care facility in a temperature- (22±2°C), humidity (50%), and light (12:12-hr light-dark) controlled room. Tap water and standard chow (Purina Mills, St Louis, MO, USA) were given ad libitum. All mice were weighed weekly.
2. Study design and exercise protocol
An overview of the experimental exercise protocol is shown in (Fig. 1). Following acclimation for a week, all mice aged 14 months were randomly assigned to the following groups: (1) CON (N=6); (2) EX_MHV (N=7); and (3) EX_MLV (N=7). The mice in exercise groups performed treadmill running on a motor-driven treadmill (Columbus Instruments, Columbus, OH, USA). Mice were acclimated to treadmill running up to 30 min at 5 to 10 m/min, 5 days prior to the initiation of the study. For the entire 8-week experimental period, EX_MHV group maintained a moderate-intensity treadmill running 5 days/week at a fixed speed of 10 m/min for 60 min, whereas EX_MLV groups engaged in moderate-intensity treadmill running 5 days/week at 10 m/min for 45 min, respectively. Mice in the CON group were not exposed to treadmill exercise other than routine daily activity. Each treadmill session included a warm-up and cool-down for 5 min at 5 m/min. The mice were encouraged to run by a gentle tap on the tail; no electrical shock was used.

Schematic overview of study design and exercise protocol. (A) C57BL/6 mice underwent 1 week of familiarization with the exercise protocol at 14-months old followed by 8 weeks of treadmill exercise training 5 days/week for exercise groups or no exercise for the non-exercise (CON) group. (B) Exercise protocol for moderate-intensity with high-volume exercise (EX_MHV) and moderate-intensity with low-volume exercise (EX_MLV) groups which consists of a warm-up, main exercise protocol, and cool-down.
After completion of the exercise protocol, all mice were anesthetized by a mixture of zoletil and rompun, and were sacrificed via myocardial removal. Right femora were harvested at sacrifice and placed in 4% paraformaldehyde at 4°C for 24 hr. Femora was subsequently stored in 70% ethanol in −20°C freezer until getting scanned by dual energy X-ray absorptiometry (DXA) and micro-computed tomography (μCT). Heart, epididymal fat, and skeletal muscles, including gastrocnemius and soleus, were also harvested and weighed at a sacrifice to show the effectiveness of the current exercise protocol.
3. DXA scan
UltraFocus DXA by Faxitron (UltraFocus DXA by Faxitron; Faxitron Bioptics, LLC, Tucson, AZ, USA) with a small animal-specific software was utilized to perform DXA scan following the tissue harvest. Whole femora were scanned and analyzed to measure the BMD and BMC.
4. μCT imaging and analyses
The femur was dissected free of skin and muscle and evaluated using a SkyScan1172 high-resolution μCT imaging system (Bruker, Kontich, Belgium). Scans were obtained at an image resolution of 10 μm, with the following settings: 1 mm aluminum filter, 65 kVP X-ray voltage, 153 μA anode current, 65 ms exposure time, 5 frames averaging, and 0.3 degrees rotation step. Trabecular bone microarchitecture parameters in the distal femoral metaphysis were determined as previously described.[8] Briefly, 60 slices were made in the distal femoral metaphysis, beginning 600 μm superior from the growth plate. Trabecular bone microarchitectural parameters including BV/tissue volume ratio (BV/TV, %), trabecular thickness (Tb.Th, μm), trabecular number (Tb.N,/mm2) and trabecular separation (Tb.Sp, μm) were calculated. For cortical bone parameters analyses, 50 slices at the femoral mid-shaft were chosen and the cortical BV (Ct.BV, mm3), cortical bone area (Ct.Ar, mm2), cortical cross-sectional thickness (mm), and polar moment of inertia (pMOI, mm4) were determined. Three-dimensional (3D) reconstruction images were generated by the Feldkamp’s algorithm using a commercially available NRecon software package (2.0.4.0 SkyScan; Bruker). The 3D morphometric analyses and imaging were performed using CTAn and CTVox software (version 1.13; Bruker).
Statistics
One-way ANOVA was utilized for parametric data analyses to examine differences among groups, followed by a post-hoc Tukey’s test. Experimental sample sizes presented in Figure 2 (BMD) and 3 (BV/TV, %) were pre-examined by using G*Power version 3.1.9.2 software (Heinrich- Heine-Universität Düsseldorf, Düsseldorf, Germany) with an anticipated effect size (F) of 0.72, which was determined based on previously published mouse hindlimb bone with aerobic exercise data.[21] With the scenario of 6 replicates per group, the difference among groups via one-way ANOVA would provide 80% power to detect effect sizes of 0.92, assuming a 2-sided significance level of 0.05. All statistical analyses were performed using GraphPad Prism version 6.01 software (GraphPad Software, Inc., San Diego, CA, USA). Quantitative data are expressed as mean±standard deviation, with P value less than 0.05 considered significant. Tendencies for significant differences (P<0.10) were reported.

Long-term moderate-intensity aerobic exercise preserves bone mineral density (BMD) in femora of middle-aged mice. (A, B) Quantification of BMD and bone mineral content (BMC) of femora. (A) Femoral BMD were significantly higher (P<0.05) in mice with moderate-intensity with high-volume exercise (EX_MHV) compared to non-exercise (CON). (B) Femoral BMC did not differ among groups. Values are means±standard deviation. *Denotes significant difference vs. CON (P<0.05). EX_MLV, moderate-intensity with low-volume exercise.
RESULTS
1. Animal body masses and tissue weights following long-term exercise training
Pre- and post-exercise body masses and tissues weights following 8 weeks of exercise training were examined (Table 1). Here, animal body masses did not differ among groups at baseline. However, it was confirmed that the body masses after exercise decreased significantly (P<0.01) in mice in EX_MHV and EX_MLV compared to CON. The effectiveness of the exercise training was next examined by using the weights of harvested tissues. Heart weight was not different among groups. However, soleus muscle mass was higher (P<0.05), and gastrocnemius muscle mass was tended to be higher (P<0.10) in the EX_MHV and EX_MLV groups compared to CON (Table 1). Similarly, epididymal fat weight was significantly lower (P<0.05) in all exercise groups (EX_MHV and EX_MLV) vs. CON. Thus, long-term aerobic exercise training leads to an increase in soleus muscle and a reduction in epididymal fat mass in middle-aged mice.
2. BMD, but not BMC, is preserved following high volume, moderate-intensity aerobic exercise in middle-aged mice
To examine the role of various volumes of moderate-intensity exercise programs in the prevention of age-related BMD and BMC loss, we first determined the BMD and BMC of femora in middle-aged mice subjected to EX_MHV and EX_MLV programs over 8 weeks (Fig. 1A, B). BMD was 8.8% higher (P<0.05) post-training in the EX_MHV group compared to CON, but EX_MLV group did not differ from CON (Fig. 2A). On the contrary, BMC did not differ among groups following moderate-intensity aerobic exercise training (Fig. 2B). Thus, long-term high volume moderate-intensity treadmill exercise is the effective method to preserve the long bone BMD during the aging process, while a low volume of moderate-intensity exercise does not achieve the desired effect.
3. Aging-associated distal femoral trabecular bone loss is mitigated following long-term moderate-intensity aerobic exercise
Next, we set out to determine the effectiveness of long-term moderate-intensity aerobic exercise in the prevention of age-associated bone loss. Meaningful differences in distal femoral trabecular bone microarchitecture were observed in the EX_MHV and EX_MLV groups compared with CON. The 3D μCT reconstructions of trabecular bone microarchitecture in the secondary spongiosa of the distal femoral metaphysis (Fig. 3A–C) demonstrated an attenuated age-related bone loss in the EX_MHV and EX_MLV groups in comparison to CON. It was confirmed by a significantly increased femoral BV/TV (43% and 40% greater among EX_MHV and EX_MLV, respectively vs. CON; P<0.05) (Fig. 3E) following moderate-intensity exercise training. Improved BV/TV in EX_MHV group was mainly affected by significantly increased femoral Tb.Th (P<0.05) (Fig. 3G) and a tendency for decreased femoral Tb.Sp (P=0.07) (Fig. 3H). On the other hand, a tendency for higher BV (P=0.08) (Fig. 3D) was the main cause of improved BV/TV in EX_MLV group. Femoral Tb.N did not differ among groups (Fig. 3F). Thus, varying volumes of long-term moderate-intensity aerobic exercise influences trabecular bone microarchitecture in the prevention of age-related declines in BV following exercise.
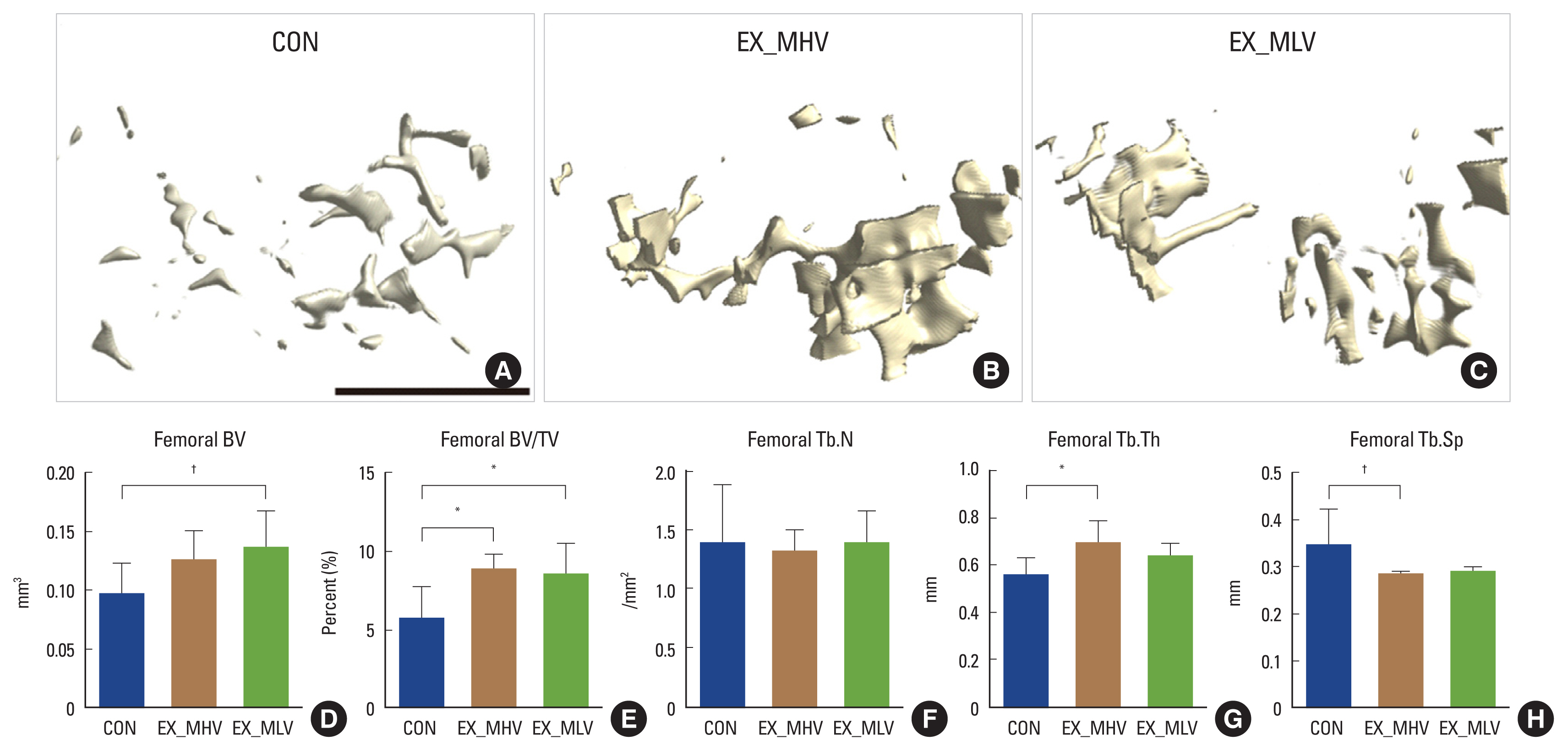
Age-associated distal femoral trabecular bone loss is attenuated by long-term moderate-intensity aerobic exercise. (A–C) Representative images of distal femoral trabecular bone microarchitecture in (A) non-exercise (CON), (B) moderate-intensity with high-volume exercise (EX_MHV), and (C) moderate-intensity with low-volume exercise (EX_MLV) mice. (D) Femoral bone volume (BV) tended to be higher (P=0.08) in EX_MLV group vs. CON. (E) Femoral BV/tissue volume (TV) was significantly greater in mice with EX_MHV and EX_MLV compared to CON (P<0.05). (F) Femoral trabecular number (Tb.N) did not differ among groups. (G) Femoral trabecular thickness (Tb.Th) was greater in EX_MHV than CON (P<0.05), but not in EX_MLV. (H) Femoral trabecular separation (Tb.Sp) was trending toward being significantly smaller in mice with EX_MHV compared to CON (P=0.09). Black scale bar: 1 mm. Values are means±standard deviation. *P<0.05. †P<0.10.
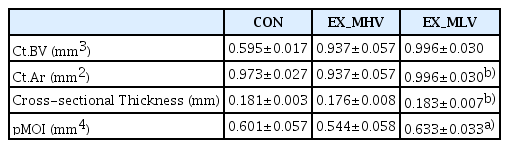
4. Effect of long-term moderate-intensity aerobic exercise on mid-diaphysis cortical bone in femora
We next determined the effect of various volumes of moderate-intensity aerobic exercise on mid-diaphysis cortical bone parameters (i.e., Ct.BV, Ct.Ar, cross-sectional Thickness, and pMOI) in femora. A significant difference in pMOI between groups was observed, whereby EX_MLV showed significantly higher (P<0.05) pMOI than EX_MHV following the exercise program (Table 2). Moreover, Ct.Ar and cross-sectional thickness tended to be greater in EX_MLV than EX_MHV (Table 2).
DISCUSSION
Current data indicate that long-term moderate-intensity aerobic exercise training, overall, beneficially preserves BMD and attenuates age-associated trabecular bone loss in the distal femora of middle-aged male mice. Moreover, we show that 8-weeks of moderate-intensity exercise training increased soleus and gastrocnemius muscle mass and reduced epididymal fat. These hypotheses generating data highlight the potential of moderate-intensity exercise training as a robust non-pharmaceutical therapy for the prevention of osteoporosis.
Osteoporosis is a common but destructive age-related metabolic disease caused by low BMD and decreasing BV.[1] Dysfunction or dysregulation of bone remodeling leads to the reduction in bone microstructure, which further causes an increased risk of fracture.[1] Incidence of bone fracture increases approximately 3.5 fold in the elderly over 60 years of age compared to a young population aged 15 to 24 years,[1,22] likely caused by a ~30% and ~41% reduction in BMD and BMC, respectively.[23,24] Moreover, preclinical evidence suggests that aging is associated with a 57% reduction in osteoblast activity and a 40% reduction in bone mineralization, thereby leading to lower bone cell turnover, resulting in ~53% lower BV in the distal femoral trabecular bone microarchitecture.[25] Although the pharmacological treatment options could lead to some side effects,[7,8,26] the development and use of a number of pharmacological therapies have led to the main treatment options to treat multifactorial processes of osteoporosis progression. For example, iPTH administration is a common therapy for treating osteoporosis.[10] Moreover, antiresorptive medication denosumab is a novel treatment drug for osteoporosis that hinders receptor activator of nuclear factor-κB ligand (RANKL), which reduces the binding of RANKL to osteoclast receptor, RANK, so that osteoclast-mediated bone resorption is lowered.[9] Nonetheless, alternative non-pharmacological therapeutic options, such as exercise and diet, are warranted. Specifically, exercise training has emerged as a prominent non-pharmacochemical option that effectively improves bone remodeling and regeneration by stimulating bone remodeling cellular responses to mechanical forces.[12] Despite this need, evidence around the optimal exercise regimen for the prevention of osteoporosis remains equivocal.
Various durations and intensities of aerobic exercise training (i.e., treadmill running, swimming, etc.) for 8 to 14 weeks have been shown to have differential outcomes in bone quality, bone mass, and BV in rodents.[18,19,27–29] One group found that total femoral BMD of young male Wistar rats was improved by ~8% following 10 weeks of high-intensity treadmill running,[18] while others found no differences in total BMD and BMC following 8 weeks and 6 months of moderate-intensity exercise.[28,29] Moreover, 8 weeks of swimming training (90 min/d, 5 day/week) in young female Sprague Dawley rats showed a 15% and 29% enlargement of tibial diaphysis cross-sectional area and moment of inertia, respectively, whereas total and cortical volumetric BMD did not change after the exercise.[27] Similarly, inconsistencies exist as to whether aerobic exercise enhances trabecular bone microarchitecture and mid-diaphysis cortical parameters in the hindlimb long bones. For instance, there is some evidence to suggest that 8 weeks of moderate-intensity aerobic exercise results in no changes in trabecular bone microarchitecture and cortical bone geometry [28]; while there is also some evidence of ~33% improvements in distal femoral trabecular bone following 10 weeks of high-intensity aerobic exercise training in rats.[18] The discrepancies in the previous findings may represent distinct physiological responses among the different bone regions of interest and aerobic exercise training modes.
The protective role of long-term exercise against chronic diseases such as osteoporosis, diabetes, and cardiovascular diseases, as well as beneficial outcomes of long-term exercise on disease conditions have been documented, thereby showing improvement in health status.[30] The current study agrees with this notion supporting the importance of long-term moderate-intensity aerobic exercise in preventing osteoporosis progression in middle-aged mice. In the present study, hindlimb BMD was significantly preserved with the high volume of moderate-intensity exercise, and age-related distal femoral trabecular bone loss was attenuated by various volumes of moderate-intensity aerobic exercise-trained mice. The mechanism by which moderate-intensity aerobic exercise can stimulate an increase in bone metabolism and bone remodeling may be related to prolonged periods of mechanical force, even during the moderate-intensity treadmill exercise, which facilitates dynamic changes of bone interstitial fluid flow.[31] Indeed, changes in the flow of interstitial fluid by mechanical loading further promote osteoblasts and osteoclasts activation and stimulates osteoprotegerin.[12–14] In addition, alterations in bone interstitial fluid flow and pressure initiate shear stress within bone, which stimulates the release of osteoblast differentiation factors, such as NO and prostaglandin E2 (PGE2).[32] For example, NO and PGE2 secretion from the osteoblasts were considerably increased with fluid shear stress over 12 dynes/cm2 compared to no fluid shear stress.[33] Upregulation of NO resulted in the 3-fold increased osteoblasts differentiation in the L-arginine-dependent pathway,[34] whereas increased NO inhibits osteoclasts-mediated bone resorption by 56%.[35] Also, PGE2 significantly augmented osteoblast markers, alkaline phosphatase (ALP), and osteocalcin (OCN). While direct measurement of PGE2 and NO were beyond the scope of the current investigation, these 2 mechanisms likely stimulated the changes in BMD seen following moderate-intensity exercise training. Thus, the profound changes in BMD and distal femoral trabecular bone following different volumes of moderate-intensity aerobic exercise training for 8 weeks, may be attributable to the increased mechanical force-induced osteoblast recruitment and reactivity.
In the current investigation, we hypothesized that moderate-intensity aerobic exercise for 8 weeks would improve cortical bone parameters in the mid-diaphysis of long bones, allowing for augmenting the strength of long bones as a training effect. However, contrary to our hypothesis, cortical bone parameters, including cortical volume, area, cross-sectional thickness, and pMOI following moderate-intensity aerobic exercise, did not differ from the non-exercise group. Discrepancies may relate to the intensity and volume of aerobic exercise. Cortical bone strength gain may be more susceptible to weight-bearing exercises such as jumping and landing instead of treadmill aerobic exercise.[20] For example, the cross-sectional area and pMOI in cortical bone of femora were higher by 15% in 8 weeks of step jumping exercise in rats compared to treadmill exercise.[20] Thus, given its similarity among groups, moderate-intensity aerobic exercise is less suitable for improving the cortical bone strength than a jumping and landing exercise regimen.
The current study has some limitations worthy of discussion. Firstly, the omission of a young control group limits our ability to strictly describe whether moderate-intensity exercise attenuated age-associated bone loss in the distal femoral trabecular bone. However, in comparing non-exercise mice with mice that performed exercise, the same species, strain, and age offset the confounding variables, which support the notion that moderate-intensity exercise improved femoral BMD and trabecular bone microarchitecture. Second, due to the limitations of the methodology applied herein, we were not able to assess the mechanical force-derived bone cellular activity and recruitment, including osteoblast and osteoclast activity and recruitment. Future work should include bone histomorphometry analysis with Goldner’s trichrome and immunohistochemical analysis of ALP or OCN to confirm the osteoblast activity and recruitment following moderate-intensity exercise.
In conclusion, the current results demonstrate that femoral BMD is beneficially preserved following 8 weeks of high volume moderate-intensity but not the low volume of moderate-intensity aerobic exercise training. Age-related trabecular bone loss was mitigated by various volumes of moderate-intensity exercise, which is presumably stimulated by increased mechanical force-derived osteoblast activity and recruitment in the distal femur. This investigation highlights the clinical impact of long-term moderate-intensity treadmill exercise as a non-pharmacological therapy for preventing bone loss and osteoporosis progression in the elderly.
Notes
The abstract of this study has been presented as the Annual Meeting of the American Society of Bone and Mineral Research (ASBMR) (1–4 Oct. 2021).
Ethics approval and consent to participate
All animal studies were approved by the Institutional Animal Care and Use Committee at Sungkyunkwan University School of Medicine (SKKUIACUC 18-5-24-3) and followed the AAALAC International Guidelines for animal experiments.
Conflict of interest
No potential conflict of interest relevant to this article was reported.