Mechanical and Mechanosensing Properties of Tumor Affected Bone Cells Were Inhibited via PI3K/Akt Pathway
Article information
Abstract
Background
Osteolytic metastasis is a common destructive form of metastasis, in which there is an increased bone resorption but impaired bone formation. It is hypothesized that the changed mechanical properties of tumor affected bone cells could inhibit its mechanosensing, thus contributing to differences in bone remodeling.
Methods
Here, atomic force microscopy indentation on primary bone cells exposed to 50% conditioned medium from Walker 256 (W) carcinoma cell line or its adaptive tumor (T) cells was carried out. Nitric oxide levels of bone cells were monitored in response to low-magnitude, high-frequency (LMHF) vibrations.
Results
A stronger sustained inhibitive effect on bone cell viability and differentiation by T cells as compared to that of its cell line was demonstrated. This could be attributed to the higher levels of transforming growth factor-β1 (TGF-β1) in the T-conditioned medium as compared to W-conditioned medium. Bone cell elastic moduli in W and T-groups were found to decrease significantly by 61.0% and 69.6%, respectively compared to control and corresponded to filamentous actin changes. Nitric oxide responses were significantly inhibited in T-conditioned group but not in W-conditioned group.
Conclusions
It implied that a change in cell mechanical properties is not sufficient as an indicator of change in mechanosensing ability. Moreover, inhibition of phosphoinositide 3-kinase/Akt downstream signaling pathway of TGF-β1 alleviated the inhibition effects on mechanosensing in T-conditioned cells, further suggesting that growth factors such as TGF-β could be good therapeutic targets for osteoblast treatment.
INTRODUCTION
Tumor metastasis to the bone is a fatalistic complication occurring in 60% to 70% breast cancer patients i.e., 5-year survival rate falls from 90% to less than 10%.[1] Osteolytic metastasis represents a common destructive form of metastasis, in which there is increased bone resorption but impaired bone formation.[23] An in vivo observation has been made of the decrease in osteoblast number and bone formation,[4] as well as lack of healing after bisphosphonate treatment.[5] This highlighted the importance of examining different mechanisms in which osteoblasts may be adversely affected by breast cancer and there has been little understanding.
Osteoblast cells were found to exhibit an increase in apoptosis [6] and reduced differentiation [78] after exposure to tumor cell conditioned medium. Morphological changes in osteoblastic cells from cuboidal to elongated spindle-like morphology were observed,[8] with punctuated filamentous actin (f-actin) fibres [79] and reduction in focal adhesion plaques.[9] LY294002, a phosphoinositide 3-kinase (PI3K)/Akt inhibitor, has been shown to maintain the morphology of breast cancer conditioned bone cells, demonstrating that induced morphology changes were mediated through PI3K pathway.[9]
However, there was no prior report on the mechanical and mechanosensing property changes of tumor affected bone cells, which could be important in developing biomechanical strategies for treatment. Mechanosensing which is sensing of mechanical signals leading to biochemical responses [10] plays a vital role in bone cells functionality [1112] and thus tissue level adaptation to mechanical loading.[13] Changes in bone cell mechanical properties including elasticity could impact its mechanosensing [14] and also be used to reflect physiological state of cells.[1516] Atomic force microscopy (AFM) with piconewton resolution could be used to probe and quantify cell elasticity.[17]
Osteoblastic cells have been demonstrated to express osteogenesis related factors with exposure to low-magnitude, high-frequency (LMHF) vibrations.[18192021] LMHF vibrations could be of interest as a potential interventional treatment, since it improved fracture healing in osteoporotic animals.[22] Nitric oxide is important in the signaling of bone cells for the regulation of bone remodeling and the addition of an inhibitor of nitric oxide also inhibited mechanically induced bone formation in rodents.[23] Thus, monitoring of nitric oxide levels in response to LMHF vibrations could be used to reflect changes in mechanosensing and degree of bone cell activation to mechanical stimuli.
This is the first paper to look into mechanical and mechanosensing changes of tumor affected bone cells, as well as the possible differences in tumor cell line and its adaptive tumor (T) cells on the bone cells. Here, we determined whether tumor conditioned medium adversely affects the mechanical and mechanosensing properties of bone cells. The inhibitory effects of T cells and tumor cell line on proliferation, differentiation (alkaline phosphatase [ALP] activity, mineralization, collagen type I deposition) and f-actin structure of bone cells were also studied.
METHODS
1. Tumor model
In the present study, 33 Sprague Dawley (SD) female rats, 8 to 10 weeks old (Laboratory Animal Centre, National University of Singapore) were housed at 25℃ with a 12:12 hr light-dark cycle and were given standard rodent diet (Model T.2018S; Harlan Laboratories, Indianapolis, IN, USA) and water ad libitum. Tumor inoculation of 107 Walker 256 (W) breast carcinoma cells (CCL-38; ATCC, Manassas, VA, USA) in 0.1 mL M199 culture medium (Sigma-Aldrich, St. Louis, MO, USA) into the medullary cavity of left femur were performed, while the right femur was kept intact (n=30).[24] Three remaining rats were kept intact for bone cell harvesting. Rats were subjected to in vivo X-ray scanning (Kodak DXS 4000 Imaging System; Carestream Health, Rochester, NY, USA) at 35 kV for 2 min under anesthesia before sacrifice at 50 days. All animal experiments were conducted in accordance with an approved protocol from Institutional Animal Care and Use Committee at the National University of Singapore.
In the tumor group, there was soft tissue tumor formation, from which tumor tissue was excised. The explants were sliced into smaller pieces under sterile conditions and placed in 6-well plates with incubation using M199 medium supplemented with 5% horse serum. The plates were left undisturbed, with minimal changes of medium during initial phases of growth.[25] The heterogeneous co-culture of explants-derived host cells with W cells were used to derive the T cells. The corresponding tumor-burdened bone preserved in 10% formalin and sent for histological sectioning and staining with hematoxylin and eosin (H&E). Signs of tumor proliferation and bone response in histological slides were examined using Olympus IX-71 Microscope.
Intact bones from 3 SD rats harvested and sectioned into smaller pieces and incubated with osteogenic medium (Dulbecco's modified Eagle's medium [DMEM]; Life Technologies, Carlsbad, CA USA) supplemented with 10% fetal bovine serum (FBS) (HyClone FBS; Thermo Scientific Inc., Waltham, MA, USA), 1x antibiotic-antimyosin (PAA) and 100 µM L-ascorbate (Sigma-Aldrich). The bone explants were maintained undisturbed for 3 to 4 weeks, until confluency of bone cells was achieved. Following which, the bone cells were trypsinized for further tests.
2. Characterization of tumor cells
Soft agar assay which tests for anchorage independent growth, is used for detecting in vitro cellular transformation and predicting potential for metastasis in vivo.[26] Soft agar assay was carried out for W and T cells and maintained at least for 2 weeks. In brief, 0.6% bottom agar is prepared by mixing 1.3 mL of 1.8% agar with 2 mL of 2x M199 and 0.7 mL of double distilled water. They were maintained at a temperature of 42℃ and plated in tissue culture plate to solidify. 0.3% top agar is similarly prepared, by mixing equal volume of 0.6% agar with equal volume of cells suspension (20,000 cells/mL) at 37℃. The top agar was left to solidify before being placed in the incubator.
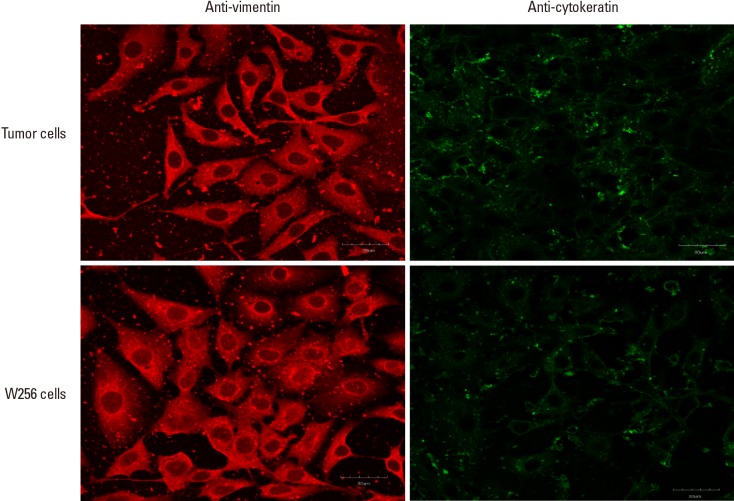
Immunostaining for vimentin and cytokeratin expression in W and T cells were also conducted. 2×104 W and T cells were seeded respectively into 24-well plates and at least 50% confluency was reached before cells were fixed with absolute methanol for 10 min in room temperature. Non-specific binding sites were blocked with 1:5 FBS in phosphate buffered saline (PBS) for 20 min. Incubation with primary antibodies i.e., anti-vimentin produced in goat (V4630; Sigma-Aldrich) at 1:100 and anti-pan cytokeratin produced in rabbit (C2562; Sigma-Aldrich) at 1:100 for an hr was then carried out. PBS was used to wash the wells for 3 times. Incubation with appropriate secondary antibodies anti-goat IgG-TRITC (Sigma-Aldrich) and anti mouse IgG-FITC (Sigma-Aldrich) at 1:100 and 1:32 respectively for an hr was carried out. PBS was again used to wash the wells and to incubate the wells for fluorescence imaging using Olympus FV1000 confocal system.
3. Conditioned medium preparation and conditioned treatment of bone cells
W and the T cells were individually cultured in M199 with 5% Horse serum and replaced with DMEM (5 mL in T-25 flask, for cell concentration of 2×106 cells/mL) for 24 hr. W and T-conditioned mediums were collected, with supernatant stored in aliquots at −20℃. Ten percent FBS and 100 µM L-ascorbate were added to the treatment medium prior to treatment of bone cells. Bone cells were treated with 50% of either W or T-conditioned medium and no treatment for control cells (i.e., 100% osteogenic medium).
W and T-conditioned mediums (without FBS) were assayed for transforming growth factor-β1 (TGF-β1) production. Culture medium on the third day of culture from respective T-conditioned and W-conditioned wells were also collected and centrifuged for supernatant. Activation of latent TGF-β1 and measurement in duplicates were carried out according to instructions in Rat TGF-β1 Quantikine enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA) (n=3). Osteogenic culture medium was similarly treated and measured as control.
4. Growth inhibition and ALP assays
For growth inhibition assays, 2×103 cells were seeded into 96-well plates and left to culture for 3, 5, and 7 days after addition of conditioned or control medium. Next, 10 µL of MTT (5 mg/mL in PBS) was added to each well and after 4 hr, the medium was removed and 100 µL of dimethyl sulfoxide added. Absorbance values at 595 nm were read on the microplate reader. Growth inhibition rates (%) were calculated as follows:
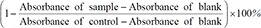
To assess ALP activities, 2×104 cells were seeded onto 24-well plates and left to culture for 3, 5, and 7 days after addition of conditioned or control medium. ALP activity was monitored with incubation of confluent bone cells with 200 µL pNPP liquid substrate (Sigma-Aldrich) for 30 min and with 50 µL of 3N NaOH solution added to stop the reaction. Absorbance measurements at 405 nm were made using a microplate reader. Inhibition rates (%) were calculated using Equation (Eq; 1).
5. In vitro mineralization and collagen type I staining
For in vitro mineralization, Von Kossa assay was carried out. Here, 2×104 cells were seeded onto a 24-well plate and after exposure to conditioned or control medium for 7 days, the medium was replaced with fresh osteogenic medium with addition of 10 mM sodium-glycerophosphate (Sigma-Aldrich) for 2 weeks. The cells were then washed with PBS and stained with 1% Silver Nitrate (Sigma-Aldrich) under UV exposure for 40 min. The wells were washed with distilled water and 3% sodium thiosulphate was added for 5 min, before being removed and washed again. Bright field images were captured using optical inverted microscope (Olympus IX-71 Microscope).
Similarly, for collagen type I staining, after exposure to conditioned or control medium for 7 days, the medium was replaced with fresh osteogenic medium for 3 weeks. Fixation of cells were first carried out using ice cold methanol (−20℃). Three-percent of bovine serum albumin solution was added to each well for 30 min, before being removed and washed. Two hundred microliters of mouse anti-collagen I antibodies in 1:1,000 dilution (C2456; Sigma-Aldrich) was added to each well and incubated for 90 min at room temperature. PBS was used to rinse the wells for 3 times. Two hundred microliters of goat anti-mouse antibody in 1:400 dilution (Alexa Fluorophore 594; Life Technologies) was added in the dark together with 4′,6-diamidino-2-phenylindole (DAPI) stain and incubated for 45 min at room temperature. Each well was rinsed 3 times with PBS and a final amount of 300 µL of PBS was added to each well before viewing using fluorescence microscopy.
6. Cell morphological and mechanical property studies
The 2.5×103 cells were first cultured for 3 days with conditioned medium or control medium in a 24-well plate. The cells were then fixed using 4% paraformaldehyde (Sigma-Aldrich), followed by labeling with 0.1 µg/mL TRITC-Phalloidin (Sigma-Aldrich) for f-actin and 5 µg/mL DAPI (Sigma-Aldrich) for cell nucleus. Fluorescence imaging was then conducted using Olympus FV1000 confocal imaging system. Cell morphology analysis (n=7 cells per group) was conducted using Image J software (NIH, Bethesda, MD, USA) by determining projected cell area and aspect ratio, i.e., ratio of maximum length to maximum width.[27]
To carry out AFM indentation of cells, 104 cells were seeded on sterile 13 mm glass cover slips (Heinz-Glas Kleintettau, Tettau, Germany) and grown for 3 days with conditioned or control medium. The live cells were subjected to AFM indentation using nanoscope IV multimode AFM with a PicoForce scanner (Veeco, Santa Barbara, CA, USA). The cells were kept immersed in PBS using a fluid cell throughout the experiment. For measuring cell mechanical property (n=30 cells per group), a silicon nitride cantilever of spring constant 0.03 N/m with a spherical polystyrene bead of diameter 4.5 µm adhered to the tip (Novascan Technologies Inc., Ames, IA, USA) was used for indentation at force of 200 pN and rate of 0.1 Hz.[28] The indentation was done at the centre of cell, to minimize influence from the substrate. From Hertz-Sneddon model, the force can be related to the indentation as:

where R is the radius of the spherical bead (2.25 µm) on the indent tip, h is the indentation depth, E is the elastic modulus of cell and ν is the Poisson's ratio, assumed to be 0.5 for biological samples.[162930] E can then be calculated from Eq (2).
7. Vibration protocol
The 2×103 cells were seeded into 96-well plate and treatment with or without 1 µM of LY294002 (Sigma-Aldrich) was carried out prior to addition of conditioned medium or normal osteogenic medium. The cells were subsequently incubated for 3 days, followed by serum starvation for one day with DMEM with 2.5% FBS for cell cycle synchronization before being maintained in normal osteogenic medium. Vibration was carried out for 20 min at room temperature on a vibration platform (Juvent 1000R; Juvent Inc., Somerset, NJ, USA) which provides vertical accelerations at 0.3 g, 30 Hz. The non-vibrated well-plate was placed at room temperature for the same period. The well plates were returned to the incubator at 37℃ for an hr before being quantified for nitric oxide. The nitric oxide concentration may be measured indirectly by nitrite and nitrate determination since nitric oxide quickly metabolizes to the more stable compounds of nitrite and nitrate. Briefly, 100 µL of medium from each well was removed and reacted with equal volume of Griess reagent (Sigma-Aldrich) at room temperature for 10 min. Measurement of the absorbance at 550 nm was carried out and nitrite concentration was determined from a sodium nitrite standard curve using 0 to 100 µM of NaNO2. Results were normalized with 5 sets of control experiments (on non-vibrated cells). Morphological changes of cells at the same time point, i.e., 1 hr after vibration was imaged using f-actin and DAPI staining using Olympus FV1000 confocal imaging system.
8. Statistical analysis
SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) was used to conduct 1-way analysis of variance (ANOVA) analysis to compare different groups, with P<0.05 being significant. All results were represented as mean±standard deviation except for AFM and nitric oxide experiments, which were represented as mean±standard error of the mean. For equal variances, Tukey's honestly significant difference post-hoc test was used for comparing between 6 groups and Bonferroni corrections for comparisons across 3 groups. For non-equal variances, Tamhane's post-hoc test was used.
RESULTS
1. Radiography and histological analyses
After 50 days, radiograph of cancer inoculated left femur showed a large soft tissue mass, with erosion of bone cortex nearer to distal growth plate (Fig. 1A). Elevated periosteal reaction at the proximal margin of tumor or ‘Codman's triangle’ [31] was also observed. In histological results, growth and expansion of the tumor cells into the soft tissue surrounding the bone and degradation of bone tissue at the distal growth plate was observed (Fig. 1B), concurring with radiographical results. It demonstrated the various effects of the injected W cells, which had selectively adapted to the host environment.
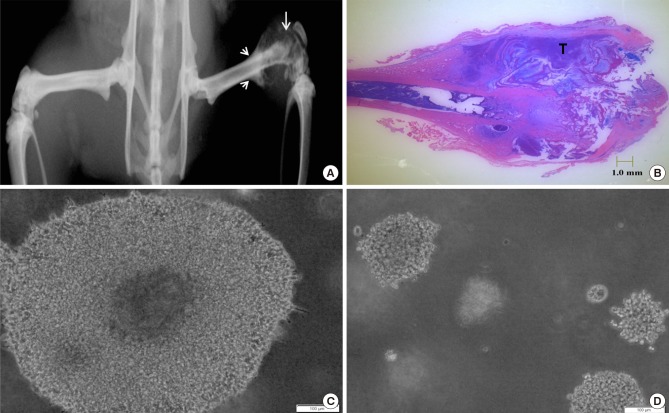
(A) Radiograph of aggressive malignant lesion (white arrow) induced with elevated periosteal reaction (arrowheads) and a soft tissue mass with aneurysmal features at 50 days. (B) Histological image of tumor burdened bone, (C) colony formation of Walker 256 cells and (D) adaptive tumor cells at 14 days, ×10 magnification.
2. Characterization of tumor cells
As observed from W and T cells colony formation, W cells formed larger colony (Fig. 1C) as compared to that of T cells with number of smaller colonies (Fig. 1D). W and T cells demonstrated anchorage independence, which is one of the characteristics for cells with metastatic potential.[26]
There was intense positive staining for vimentin in both T and W cells (Fig. 2), with light or minimal staining for cytokeratin expression. Immunophenotypic analysis indicated that both T and W cell types are hematopoietic in origin,[32] with minimal epithelial cell markers. This suggested that T cells derived might not have significantly changed in immunophenotype from its cell line, despite of differences in environment (being in a co-culture with host cells).
3. TGF-β1 production
TGF-β1 in acid-activated T and W-conditioned medium (without FBS) were found respectively, to be 20.2 pg/mL and 14.3 pg/mL. Acid activated medium collected on the third day of culture from T- and W-conditioned wells showed that TGF-β1 levels were 113.3 pg/mL and 65.9 pg/mL respectively, after correction for basal medium TGF-β1 levels. It appeared that there was a dose-dependent effect on TGF-β1 production by bone cells through exogenous TGF-β1 conditioning, which agreed with an earlier report.[33]
4. Growth and ALP activity inhibition
As observed from Figure 3A, T-conditioned medium induced a 2-fold higher inhibition of bone cell growth than W conditioned medium at 3 days of exposure (+12.93%, P<0.001) and at 7 days of exposure (+13.34%, P=0.008). There was no significant difference in inhibition rates for T group from 3 to 7 days. By 2-way ANOVA analysis, only the treatment medium was indicated to have significant effect (P<0.001).
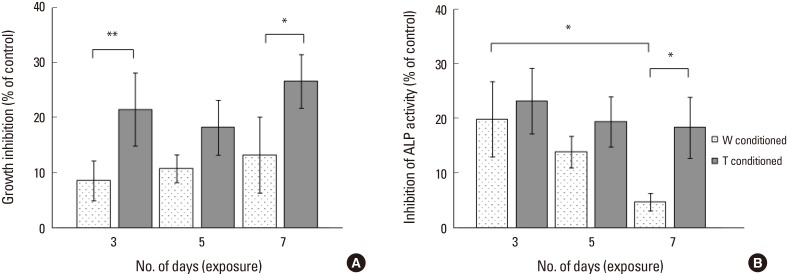
(A) Growth inhibition of bone cells and (B) alkaline phosphatase activity inhibition of bone cells by Walker 256 (W) and adaptive tumor (T) conditioned medium for 3, 5, and 7 days of exposure. *Indicated P<0.05 and **indicated P<0.001 for one-way analysis of variance analysis, with Tukey's honestly significant difference post-hoc test.
T group had a significantly higher inhibition of ALP activity than W group at 7 days exposure (+13.57%, P=0.016) (Fig. 3B). While there was no change in inhibition rate of ALP activity by T conditioned medium, there was decreasing inhibition by W conditioned medium on ALP activity from 3 to 7 days, with W group inhibition rate at the seventh day being significantly lower than that on the third day (−15.12%, P=0.008). The 2 main effects, i.e., number of days of exposure (P=0.002) and the treatment medium (P=0.002) were each indicated to be significant from 2-way ANOVA analysis.
5. Mineralization and type I collagen staining
Control bone cells (Fig. 4A) formed brown stained mineralized nodules, which were qualitatively smaller for W conditioned bone cells (Fig. 4B) and observed to be absent for T conditioned bone cells after 7 days of exposure (Fig. 4C). The degree of mineralization was also reduced, as seen from the lower presence of brownish stains.
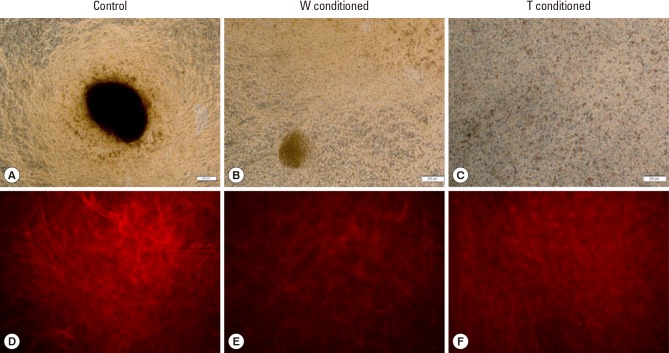
(A) Von Kossa staining of control well, (B) Walker 256 (W)-conditioned well and (C) adaptive tumor (T)-conditioned well at ×4 magnification, scale bar: 200 µm. (D) Collagen I staining of control well, (E) W-conditioned well and (F) T-conditioned well at ×20 magnification, small scale bar: 17 µm, large scale bar: 96 µm.
The control bone cells tended to form more distinct fiber organization (Fig. 4D) than W conditioned (Fig. 4E) and T conditioned bone cells (Fig. 4F). For T conditioned bone cells, the type I collagen formed were observed to be more amorphous, without any distinctive fiber organization.
6. Morphological changes
Control bone cells retained their cuboidal morphology with dense f-actin fibers (Fig. 5A), with mean cell area 2,322±1,362 µm2 and aspect ratio 1.68±0.46. Exposure to W and T conditioned medium for 3 days induced morphology changes, with reduced cell area (1,158±481 and 1,004±614 µm2 respectively for W and T groups) and significant elongation i.e., higher aspect ratio 3.55±1.67 (P=0.046) and 2.71±0.917 (P=0.036) for W- (Fig. 5B) and T groups (Fig. 5C) respectively. Density of f-actin fibers was observed to be reduced across centre of cell in W group and punctuated stress fibers of bone cells in T group were noted. F-actin fluorescence levels across groups were quantified using micro-plate reader (data not shown) and no significant differences were observed. Thus, changes of cytoskeleton occurred in the reorganization of f-actin fibers and not as a reduction of f-actin expression levels.

(A) F-actin staining of control well, (B) Walker 256 (W)-conditioned well and (C) adaptive tumor (T)-conditioned well at ×40 magnification, scale bar: 50 µm. Cell nucleus was counterstained with 4′,6-diamidino-2-phenylindole. (D) Plot of indentation force vs. indentation depth, (E) Bar chart plot of elastic moduli for control, W-conditioned and T-conditioned groups. **Indicated P<0.001.
7. Elastic modulus of bone cells
It was observed that there were higher indentation depths for the same indentation load for both W- and T-groups as compared to control (Fig. 5D). In fact, Young's modulus (E) decreased significantly for W- and T-groups by 61.0% (P<0.001) and 69.6% (P<0.001) as compared to control (Fig. 5E).
8. Vibration of bone cells
Nitric oxide response of bone cells to LMHF vibrations were significantly inhibited by 78.2% (P=0.013) in untreated T-conditioned cells (Fig. 6) but not in W-conditioned cells. On the other hand, no significant difference was found between LY294002 treated T-conditioned cell from both treated and untreated control groups, indicating that nitric oxide response with treatment is similar to that of control.

(A) Bar chart plot of normalized nitric oxide levels across vibrated control, Walker 256 (W)-conditioned and adaptive tumor (T)-conditioned groups with treatment and without treatment of LY294002, *indicated P<0.05. (B) F-actin staining of vibrated control, W- and T-conditioned wells, with or without treatment of LY294002 at ×60 magnification, scale bar: 30 µm.
With vibration, LY294002 treated W-conditioned cells (Fig. 6) were morphologically similar to that of both treated and untreated control wells. However, in LY294002 treated T-conditioned wells, there were still the appearances of elongated cells as with the non-treated T-conditioned wells.
DISCUSSION
The specific aims of this study were to determine whether tumor conditioned medium adversely affects the mechanical and mechanosensing properties of bone cells and on possible differences between the inhibitory effects of T cells and tumor cell line on primary bone cells.
Both W cell line and T cells displayed similar immunophenotypic expression and exhibited anchorage independence that indicated metastatic potential. However, size difference in colony formation suggested varying tumor subpopulations being propagated. It is well understood that the selective adaptation of tumor cells and osteolysis development depended on inherent tumor characteristics to form symbiotic interactions with host cells in the bone microenvironment.[34] As such, T cells having interacted with host cells could have selectively different characteristics from that of the immortalized tumor line. Therefore, it would be pertinent to examine any possible contrasts in effects of T conditioned medium on bone cells, since earlier in vitro studies have used tumor cell line conditioned medium.
Overall, it was demonstrated that there was a stronger and sustained inhibitive effect on bone cells viability, differentiation (i.e., ALP activity) as well as mineralization by tumor (T) conditioning compared to W conditioning. This could be attributed to the higher levels of TGF-β1 in the T-conditioned medium compared to the W-conditioned medium. An earlier study has corroborated with this observation, demonstrating that the presence of TGF-β in the conditioned medium of MDA-MB-231 was partially responsible for the inhibition of osteoblast differentiation.[7]
TGF-β has been shown to play both positive and negative influences over osteogenic differentiation,[353637] cell viability [3538] and its effects vary based on concentration as well as cell types.[353739] Nonetheless, it has been shown that repeated exposure to low dosage of TGF-β1 could reduce insulin-like growth factor (IGF)-1 expression and down regulation of PI3K/Akt pathway, resulting in the inhibition of osteoblast differentiation.[39] As such, the concentration differences of growth factors in different conditioned mediums could contribute to the varied inhibitive effects on bone cells.
It was further observed that there was less structural organization of collagen type I with T-conditioning that could contribute to the mineralization pattern. While collagen synthesis was reported to be downregulated in tumor affected osteoblast cells,[40] less was known about the physical organization of collagen deposits. T-conditioning was observed to result in defective bone matrix deposition i.e., amorphous collagen organization and reduced mineralization, which could adversely affect cell adhesion, mechanical and mechanosensing properties. Since type I collagen has been demonstrated to support osteoblast differentiation and maintenance of the bone cell phenotype,[41] there could be reinforced negative feedback for bone cell differentiation with T-conditioning that could be sustained over longer time.
It was shown that there was significantly reduced elasticity of the bone cells exposed to the T-conditioned medium. The actin network is demonstrated to be important for cell mechanical stability and directly affects cell elastic modulus.[283042] Thus, the observed reduction in elastic modulus could be associated with the changes in f-actin organization with tumor conditioning, where f-actin density was not only reduced across centre of cell (indentation location) but also more punctuated.
There could be another contributing factor to the altered cell stiffness, i.e., the substrate, since actin network rearranges on attachment to extracellular matrix.[43] With 7 days of exposure to W and T conditioned medium, there was inhibition in terms of the degree of mineralization and formation of bone nodules after 2 weeks. Therefore, it was possible that at the time-point of the AFM study (3 days of exposure), there could be differences in mineralization that may contribute to variation in cell cytoskeletal arrangement and thus stiffness. However, quantification of mineralization at the third day (data not shown) did not indicate a significant difference in mineralization between the groups. Thus, substrate mineralization might not contribute to the observed differences in cell stiffness.
The reduced stiffness could also be linked to inhibited differentiation, since ALP activity (an early differentiation marker) of 100% confluent bone cells were inhibited by both types of tumor conditioned medium. Indeed, distinct mechanical property differences were found between different phenotypes of differentiated cells and of undifferentiated bone marrow derived mesenchymal stem cells,[29] indicating that the variation of cell differentiation stages could be a contributing factor to mechanical property.
The ability of bone cells to detect mechanical signals and respond with appropriate biochemical signals is dependent on the cytoskeleton.[1344] For instance, through microfilament disruption, osteoblasts under cyclic compression loading were inhibited in c-Fos mRNA and protein expression that is important for bone growth and mineralization.[13] F-actin organization changes and increased deformability of T- and W-conditioned bone cells could therefore affect their mechanosensing properties and contribute to changes in remodeling events.
Interestingly, while mechanical properties of both T- and W-conditioned cells were significantly less stiff than control cells, only T-conditioned cells were significantly inhibited in mechanosensing. This implied that cell mechanical property is not sufficient as an indicator and cytoskeletal organization is not the only factor influencing the mechanosensing process of bone cells. It is possible that the presence of growth factors in the conditioned medium could serve as negative modulators of the mechanosensing process. Moreover, it was shown that bone cells at various differentiation stages have different responses to mechanical signaling.[45] Thus, inhibition of differentiation by tumor-released factors (even with 3 days of exposure) could give rise to variation of bone cell differentiation stages, with differing mechanical and mechanosensing properties.
Here, LY294002, an inhibitor of PI3K signaling pathway through which TGF-β, IGF-2 and platelet-derived growth factor mediate their effects on cytoskeleton,[9] was used at a dosage of 1 µm (IC50=1.40 µm, 50 µm completely abolished PI3K activity [46]). It was shown to alleviate the inhibition on mechanosensing properties of T-conditioned cells, which indicated the involvement of PI3K signaling pathway. It also appeared to maintain cytoskeletal organization of tumor-affected cells, which was corroborated by an earlier study.[9]
Since down regulation of PI3K/Akt pathway inhibited osteoblast differentiation [39] and mechanosensing varies with differentiation, it was not surprising that nitric oxide levels of LY294002 treated control and W-conditioned groups decreased, as compared to respective untreated groups. However, by alleviating nitric oxide levels of treated T-conditioned groups through maintenance of cytoskeleton organization, it did highlight on the importance of cytoskeleton in mechanosensing. Furthermore, it does suggest the potential of growth factors such as TGF-β and its downstream molecules as pharmacological targets for osteoblast treatment in osteolytic metastasis.
Nevertheless, there were some limitations in this study, namely experiment time-point and experimental conditions, like temperature and substrate. Three days of tumor conditioning for AFM indentations and mechanosensing was carried out and it could be interesting to examine if reduced bone cell stiffness and mechanosensing would be sustained over a longer periods of tumor conditioning. All AFM indentations were conducted on hydrated glass slips under room temperature. Similarly, vibration studies were conducted under room temperature for 20 min. For consistent comparisons, experimental durations were controlled to ensure that analyzed cells from all 3 groups remained attached during the experiment, since relative cell attachment could affect cellular mechanical and mechanosensing properties.
In our study, it was demonstrated that there was stronger sustained inhibition of bone cells viability and differentiation by primary tumor conditioned medium than the original cell line conditioned medium. This could be attributed to higher levels of TGF-β1 in the T-conditioned medium than that of W-conditioned medium. It was also demonstrated that there was significantly reduced stiffness and mechanosensing of bone cells when exposed to T-conditioned medium, in which the former could be associated with f-actin changes and the latter, possibly with inhibited differentiation and cytoskeleton changes. PI3K/Akt pathway inhibitor LY294002 at 1 µM alleviated the inhibition of mechanosensing in T conditioned cells, suggesting that growth factors like TGF-β1 could be good pharmacological targets for osteoblast treatment in osteolytic metastasis.
Acknowledgments
This work was supported by National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2019R1F1A1058182) and Ewha Womans University Research Grant of 2016.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.