The Efficacy of Bisphosphonates for Prevention of Osteoporotic Fracture: An Update Meta-analysis
Article information
Abstract
Background
The efficacy of bisphosphonates for osteoporotic fracture has been consistently reported in recent randomized controlled trials (RCTs) enrolling hundreds of patients. The objective of this study was to update knowledge on the efficacy of available bisphosphonates in the prevention of vertebral and non-vertebral fractures.
Methods
An approach “using systematic reviews” on PubMed and Cochrane Library was taken. Twenty-four RCTs investigating the effects of bisphosphonates for the prevention of osteoporotic fracture were included in final analysis. A pairwise meta-analysis was conducted with a random effects model. Subgroup analysis was performed according to the type of bisphosphonate.
Results
The use of bisphosphonate decrease the risk of overall osteoporotic fracture (odds ratio [OR] 0.62; P<0.001), vertebral fracture (OR 0.55; P<0.001) and non-vertebral fracture (OR 0.73; P<0.001). Subgroup analysis indicated that zoledronic acid showed the lowest risk reduction (OR 0.61; P<0.001) for overall osteoporotic fractures but no significance was observed for etidronate (OR 0.34; P=0.127).
Conclusions
This update meta-analysis re-confirmed that bisphosphonate use can effectively reduce the risk of osteoporotic fracture. However, there is a lack of evidence regarding etidronate for the prevention of osteoporotic fracture.
INTRODUCTION
Osteoporotic fracture is recognized as a major social health problem.[1] Patients who sustain osteoporotic fractures have higher rates of morbidity and mortality.[2] Recently, several pharmacologic treatment options including antiresorptive or anabolic agents have been continuously developed.[34] However, bisphosphonates such as alendronate, risedronate, ibandronate and zoledronate are still the mainstay of anti-osteoporotic treatment.[5]
The efficacy of bisphosphonate in postmenopausal women has been thoroughly researched and reported in many clinical trials.[678] Moreover, a number of meta-analyses of bisphosphonates have been performed, but recently there have been adverse effects related reviews and relatively few reviews on the clinical impact of fracture prevention in the overall population.[910] To date, many clinical trials have been undertaken to elucidate the impact of bisphosphonate for osteoporotic fracture prevention.
Thus, we conducted an update meta-analysis to evaluate the efficacy of bisphosphonate regarding the prevention of osteoporotic fracture in patients with osteoporosis. The purpose of our update study was to determine the incidence of (1) overall osteoporotic fracture, (2) vertebral/non-vertebral fracture between intervention and placebo group.
METHODS
1. Literature search of previous systemic reviews
This search was conducted according to the updated preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) guidelines (Appendix S1).[11] First, a comprehensive search for systematic reviews or meta-analyses comparing outcomes between bisphosphonate and placebo in patients with osteoporosis on Cochrane Library (Cochrane Database of Systematic Review) was performed on November 13, 2016. The methodological quality of the included systematic reviews was independently assessed by the first two authors using the Assessment of Multiple Systematic Reviews (AMSTAR) tool.[12] Thereafter, we identified relevant randomized controlled trials (RCT) through the examination of reference lists from selected meta-analyses.[13141516]
2. Literature search for update clinical trials
We performed an additional updated searching of primary studies on PubMed-Medline and Cochrane Library search in November 2016 using key terms (bisphosphonate OR osteoporosis OR fracture). An overview of the search strategy and general characteristics about the four included systematic reviews are summarized in Appendix S2. Inclusion criteria were as follows: (1) study was a RCT, (2) patients were above 50 years old, and (3) articles that provided the rate of osteoporotic fracture. The intervention of at least one study group included the following bisphosphonates: alendronate, etidronate, ibandronate, risedronate, clodronate minodronate, pamidronate, tiludronate or zoledronic acid. We restricted our review to English and Korean studies, owing to translation difficulties and lack of resources for review. Basic science articles, comments, and letters were all excluded. When a published, updated study involving the same cohort of patients was identified, only the latest update was included in the analysis.
3. Outcome measure and data extraction
The primary outcome of interest was the rate of osteoporotic fracture including all fractures. Osteoporotic fractures were categorized into vertebral fractures and non-vertebral fractures. Additionally, we performed a subgroup analysis according to the type of bisphosphonate.
For each selected study, the following data were extracted and entered in a spread sheet by the two reviewers: the family name of the first author, the year of publication, enrollment period, study design, number of patients, number of osteoporotic fracture, mean age at the time of intervention, and the duration of follow-up.
4. Quality assessment and publication bias
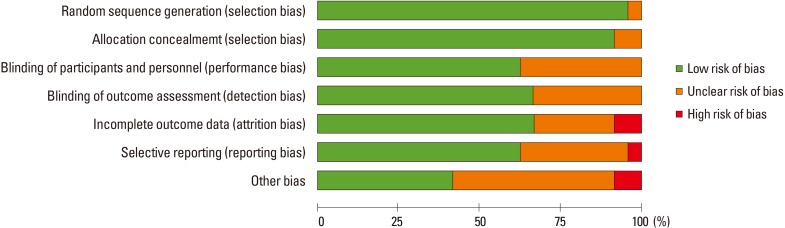
Two of the authors (BJH, YBH) independently evaluated the quality of all the studies, using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions. The criteria included the following 7 items: (1) random sequence generation (2) allocation concealment (3) blinding of participants and personnel (4) blinding of outcome data (5) incomplete outcome data addressed (6) selective reporting (7) other biases. We assessed publication bias with Begg's funnel plot and Egger's test.
5. Statistical analysis
For each study, we calculated the odds ratio (OR) with a 95% confidence interval (CI) by using crude 2×2 tables, whenever possible, from the comparative studies.[17] The Mantel–Haenszel method was used to calculate the OR due to zero values in any cell count in a table.[18] Heterogeneity between comparable studies was tested with the chi-square and Higgins I2 test. P-value of less than 0.1 or an I2 value higher than 50% meant significant heterogeneity and a random-effects model should be applied. There was significant heterogeneity between the included studies (P=0.001). Thus, we reported the data from a randomized-effect. All analyses were performed using STATA version 14.0 software (Stata Corporation, College Station, TX, USA).
This study was exempted from institutional review board review since it did not involve any human subjects.
RESULTS
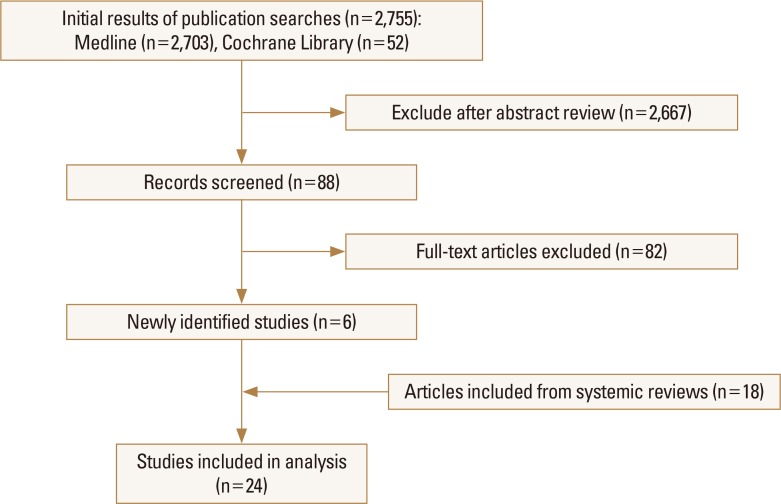
1. Description of selected trials A primary search of databases yielded
A primary search of databases yielded 2,755 records and a total of 24 studies were included in the final systematic review. From the initial searches, studies selected for final inclusion or exclusion are displayed in the flowchart (Fig. 1). Twenty-four randomized placebo controlled trials investigating the effects of alendronate (6 studies), risedronate (5 studies), etidronate (4 studies), zoledronic acid (4 studies), clodronate (2 studies), ibandronate (1 study), minodronate (1 study), and pamidronate (1 study) were identified with a systematic literature search.[678192021222324252627282930313233343536373839] The characteristics of studies included are summarized in Table 1. A final total of 39,197 patients were included in our meta-analysis: 21,335 patients in the bisphosphonate group and 17,862 patients in the placebo group.
2. The overall rate of osteoporotic fracture
The overall rate of osteoporotic fracture was 7.7% (3,036/39,197): 5.9% (1,268/21,355) in the bisphosphonate group and 9.9% (1,768/17,862) in the placebo group. The overall rate of vertebral fracture was 5.9% (446/7,585) in the bisphosphonate group and 10.3% (741/7,190) in the placebo group. The overall rate of non-vertebral fracture was 6.0% (822/13,750) in the bisphosphonate group and 9.6% (1,027/10,672) in the placebo group.
3. Efficacy of bisphosphonate for osteoporotic fracture risk reduction
In the random effects model for all studies, the use of bisphosphonate was associated with a decreased risk of osteoporotic fracture (OR 0.62; 95% CI 0.54 to 0.71 P<0.001). In vertebral fracture, the use of bisphosphonate was associated with a decreased risk of osteoporotic fracture (OR 0.55; 95% CI 0.44 to 0.69 P<0.001) and non-vertebral fracture (OR 0.73; 95% CI 0.67 to 0.81 P<0.001) (Fig. 2). In the subgroup analysis, zoledronic acid showed the lowest OR with significance for overall osteoporotic fractures (Table 2).
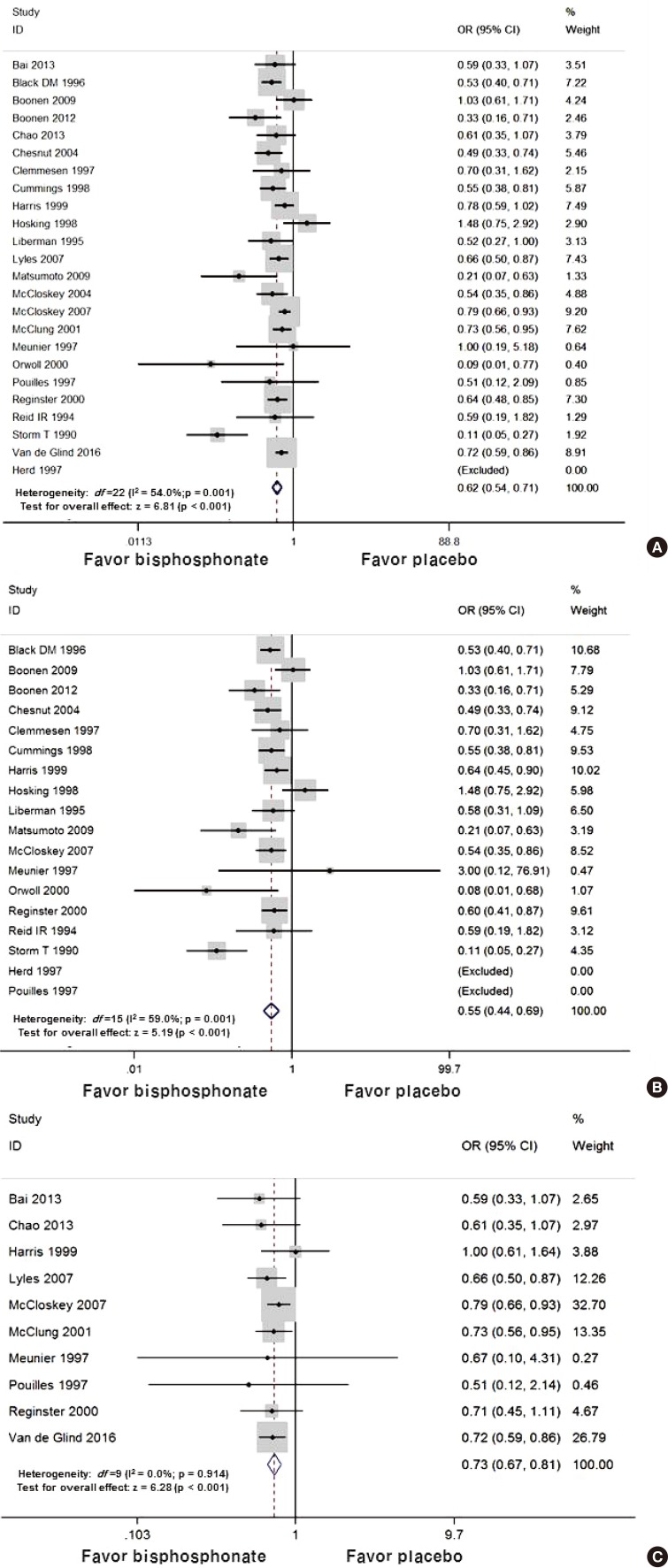
(A) The forest plot of overall osteoporotic fracture reductions relative to placebo by bisphosphonate treatment. The forest plot of odds ratio for (B) vertebral fracture. (C) non-vertebral fracture. OR, odds ratio; CI, confidence interval.
4. Quality assessment and publication bias
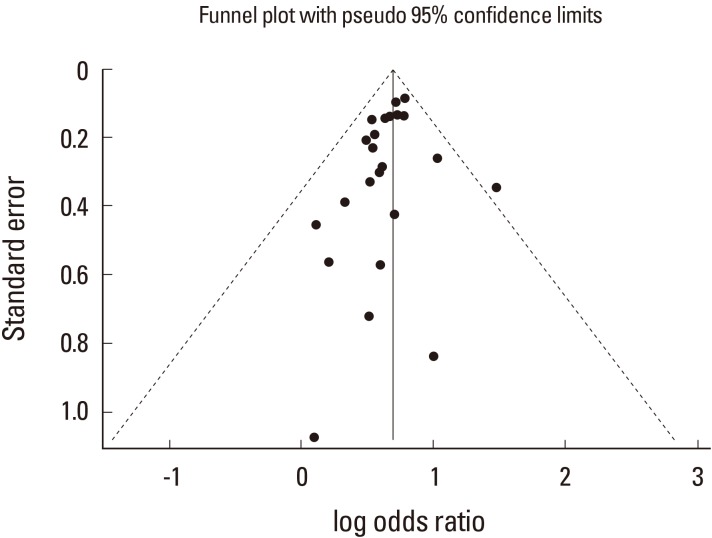
In terms of the methodological quality, subjects were randomized by established allocation sequence, and investigators and research assistants were all blind to allocation. However, it is unclear whether the included trials met all the quality assessment criteria (Fig. 3). The Begg's funnel plot was symmetrical, and P-value for bias was 0.192 in the Egger's test for the included trials (Fig. 4).
The funnel plot shows publication bias for osteoporotic fracture related to the use of bisphosphonate. There was symmetry, suggesting that there was no significant publication bias in the twenty-four studies.
DISCUSSION
One potential approach to enhance the management of osteoporosis is drug treatment that decreases osteoclastic activity.[40] Bisphosphonates are widely used as first-line drug therapy for preventing osteoporotic fracture and their efficacy is well-established. The objective of the current study was to synthesize updated evidence of bisphosphonates in the prevention of vertebral and non-vertebral fractures among patients with osteoporosis. A previous meta-analysis which included 8 RCTs reported that the use of bisphosphonates may result in anti-fracture and bone mineral density-increasing effects in postmenopausal women.[41]
Our update review included quality-proven 24 RCTs and the observed OR was 0.62 for total osteoporotic fracture and 0.55 for vertebral fracture. The effect of bisphosphonates on anti-fracture was similar to that obtained in a previous meta-analysis reporting beneficial effects of bisphosphonates in postmenopausal women. Relative risk reduction in vertebral fractures ranged from 0.55 to 0.61.[13141516] However, etidronate use did not show significant risk reduction for non-vertebral fractures.[14] This finding was similarly observed in our analysis from four RCTs (Table 2).
The efficacy of bisphosphonates varied between the included studies. A previous network meta-analysis demonstrated that zoledronic acid was most effective for reducing vertebral fractures.[41] Our study also noted the lowest OR in zoledronic acid. Improved compliance and poor gastrointestinal absorption of oral bisphosphonates are suggested as a possible explanation. Although there is a difference of efficacy between different types of bisphosphonate, the efficacy of bisphosphonate was predominantly dependent on the compliance to bisphosphonate.[42]
This study has some limitations. First, the current analysis did not involve evidence on quality of life or survival. Secondly, the potential adverse effects of bisphosphonates were not evaluated in this study. Third, hip fractures which are significantly associated with morbidity and mortality were not analyzed separately.
In conclusion, this update meta-analysis re-confirmed the efficacy of bisphosphonate in patients with osteoporosis. In terms of their anti-osteoporotic fracture efficacy, bisphosphonates except etidronate still demonstrate its effectiveness.
ACKNOWLEDGEMENT
This research was supported by a grant of the Korea Health Technology R & D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Korea (grant number : HI13C1522 and HC15C1189).
Sponsor's Role: The funding organizations had no role in the design, methods, analysis, preparation, or approval of the paper. The views expressed in this publication are those of the authors and not necessarily those of the KHIDI.
Notes
No potential conflict of interest relevant to this article was reported.
Appendix
Appendix 1
The updated checklist consists of 17-items intended to facilitate the preparation and reporting of a robust protocol for the meta-analysis and systematic review
Appendix 2
The search strategy that details the selection process of relevant clinical studies