Association between Hemoglobin Level and Bone Mineral Density in Korean Adults
Article information
Abstract
Background
Low bone mineral density (BMD) increases fracture risk. Anemia is highly prevalent. Some studies suggested that anemia is an independent risk factor for osteoporosis. The aim of this study was to evaluate the association between hemoglobin (Hb) level and BMD in Korean adults.
Methods
This study was based on data from the Korea National Health and Nutrition Examination Survey 2008 to 2011. Propensity score-matching was performed to establish a dataset for analysis. Regression analyses were used to evaluate the association between Hb and BMD. Mean BMD scores were compared between normal and anemia groups, and among normal, anemia with low ferritin, and anemia without low ferritin groups.
Results
There were partial positive associations between Hb and BMD in men, but negative associations in women. The normal group had lower mean BMD scores than the anemia group in men. However, the anemia group had higher whole-body and lumbar-spine BMD scores than the normal group in women. In a comparison of the three groups, the anemia without low ferritin group had the lowest all-site BMD scores in men and women. However, the anemia with low ferritin group had the highest all-site BMD scores in women. In postmenopausal women, the anemia without low ferritin group had the lowest all-site BMD scores. In premenopausal women, the anemia with low ferritin group had the highest whole-body BMD scores.
Conclusions
This study found that Hb and anemia status were associated with BMD scores. However, the associations were different according to sex. This may be due to the etiology of anemia or low Hb levels.
INTRODUCTION
As the populating is aging, the prevalence of osteoporosis and osteopenia associated with an increased risk of fracture are showing increasing trends.[12] This is a global health problem, and many previous studies revealed basic metabolic conditions and various risk factors associated with the risk of osteoporosis. Old age, underweight, low physical activity level, educational level, and dyslipidemia are well-known factors associated with the loss of bone mineral density (BMD).[3456] In addition, the use of steroids or excessive drinking and smoking is also known to be associated with reduced BMD.[7] Prevention and treatment for targeted people who are expected to have BMD loss due to such risk factors are essential to prevent BMD loss and associated fractures.
Anemia is a common disease. According to the “2011 National Health Statistics,” the prevalence rates of anemia in men and women aged >10 years were 2.4% and 12.7%, respectively, and the prevalence rate of anemia in men and women aged >70 years were 15.3% and 17.8%, respectively. Low hemoglobin (Hb) levels themselves are a health hazard and known as a risk factor of various diseases. Hypoxia is known to be a risk factor of osteoporosis,[8910] and studies have reported a positive correlation between Hb levels and BMD in patients with certain diseases such as sickle cell anemia, chronic obstructive pulmonary disease, inflammatory bowel disease, and chronic kidney disease.[11121314] In addition, a recent study involving older postmenopausal women reported that anemia was an independent risk factor of low BMD.[15] One hypothesis is based on the hematological etiology of the association between blood loss and BMD loss.[16] Although studies have investigated the association between Hb levels and bone density in older persons or patients with certain disease,[1718] studies that investigated the association between Hb levels and bone density in the general population are scarce. In addition, studies in which BMD was measured using dual energy X-ray absorptiometry (DXA) are limited.
The aim of the present study was to evaluate the association between Hb levels and BMD in men and women aged >20 years through various analysis methods, based on data from the fourth and fifth Korea National Health and Nutrition Examination Survey (KNHNES).
METHODS
1. Subjects
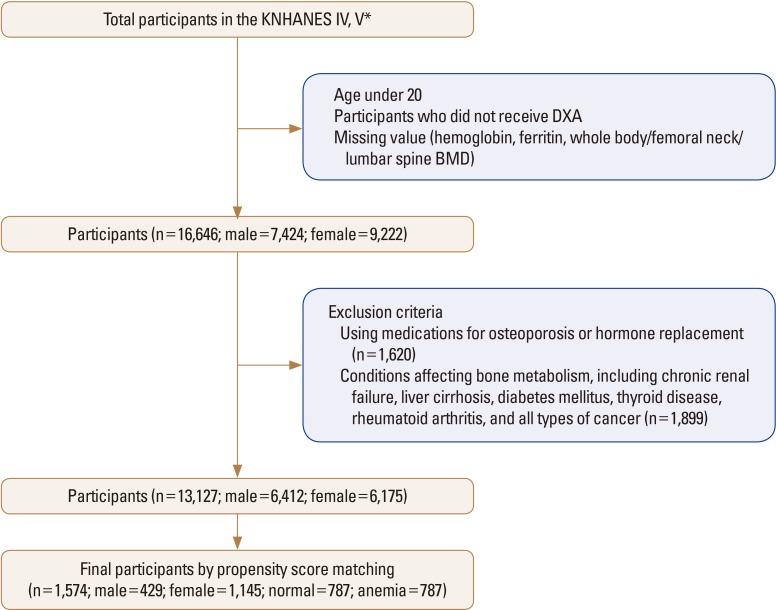
The subjects of the present study were selected based on raw data from the fourth and fifth KNHNES (2008-2011). Of all the subjects, those aged <20 years were excluded and those undergoing DXA for BMD measurement were selected. Of the selected subjects, those who did not have whole-body BMD, femoral neck BMD, lumbar spine BMD scores, or Hb and ferritin levels were excluded. A total of 16,646 subjects were primarily selected, consisting of 7,424 men and 9,222 women. Of these subjects, 1,620 patients who were currently taking osteoporosis drugs or female hormones were excluded. In addition, 1,899 patients with chronic renal failure, liver cirrhosis, diabetes mellitus, thyroid disease,[19] rheumatoid arthritis, and all types of cancer, which are known to affect bone metabolism, were excluded. Therefore, 13,127 subjects (6,412 men and 6,715 women) were finally selected. Marked differences were observed in variables, such as age, body mass index (BMI), drinking frequency, education level, and physical activity level, which could cause a difference in BMD between the normal and anemia groups among the selected subjects. Therefore, to control this, the propensity score matching (PSM) method was used to establish a dataset to ensure the homogeneity of the subjects prior to the difference verification. Typical methods that use propensity scores include stratification, matching, and inverse probability. In the present study, the technique of matching a delta value to the predictive value, or a logit score to ≤0.0003 was used to homogenously extract the normal and anemia groups in terms of covariance (age, BMI, physical activity, drinking frequency, and educational level) considered in the present study. Consequently, 1,574 subjects (429 men and 1,145 women), 787 subjects each for the normal and anemia groups, were finally selected for analysis (Fig. 1).
2. Data collection
The demographic variables (age, sex, educational level, and monthly income) and health behavior information (smoking, alcohol consumption, and physical activity level) of the subjects were collected through a survey. High-risk drinkers were defined as men consuming alcohol more than twice per week and ≥7 drinks per drinking occasion, and women consuming ≥5 drinks per drinking occasion. Physical activity level was quantified into metabolic equivalent of task-minute per week and classified into three levels by using the Korean version of the International Physical Activity Questionnaire.[20] Calcium and iron intakes (mg/day) were obtained using the 24-hr recall method.
Height (cm) and body weight (kg) were measured, and BMI was calculated, accordingly. The BMD scores of the subjects were measured using DXA. To evaluate osteoporosis and osteopenia, T-scores of ≥−1, >−2.5 but <−1.0, and ≤−2.5 in BMD testing were classified as normal, osteopenia, and osteoporosis, respectively, according to the World Health Organization (WHO) T-score criteria. In accordance with the recommendations of the International Society for Clinical Densitometry (ISCD), the lowest T-score value among the T-scores at three measurement sites (lumbar spine, whole femur, and femoral neck) was used as diagnostic criteria for osteoporosis. In view of the fact that the standard value for the maximum BMD in South Korea has not yet been established yet, it was calculated based on the maximum BMD in Asia (Japan).[21]
Hb (g/dL) and serum ferritin (ng/mL) levels were obtained through blood tests performed during the study period. Anemia was defined as Hb levels of <13 g/dL for men and <12 g/dL for women in accordance with the WHO diagnostic criteria.[22] The baseline iron deficiency status was set at a serum ferritin level of <15 ng/mL for both men and women, and the normal upper limit of serum ferritin levels was defined as 200 ng/mL for men and 150 ng/mL for women.[23]
3. Statistical methodologies
In the present study, the general characteristics of the subjects were presented as mean±standard deviation, and analyses were performed as follows: All analyses were performed using SPSS 20.0 for Windows (IBM Corp., Armonk, NY, USA). The P-values of all the results reported below were bilateral, and the significance level was set at P<0.05.
First, all the subjects were divided into three groups (normal, anemia with low ferritin group, and anemia without low ferritin groups). To compare the basic demographic characteristics between the groups, the categorical data were presented as a percentage and compared by using the χ2 test, while the continuous data were calculated as mean and standard deviation and compared through an analysis of variance (ANOVA) (Table 1). In addition, the original subjects and the subjects selected using the PSM method were divided into the normal and anemia groups to compare the characteristics of the original dataset with those of the PSM dataset (Table 2). Subsequently, to check the selection convenience problem of the subjects selected using the PSM method, the odds ratios (ORs) of the osteoporotic diseases (osteoporosis or osteopenia) were compared using the total dataset and the dataset applied using the PSM method (Table 3).
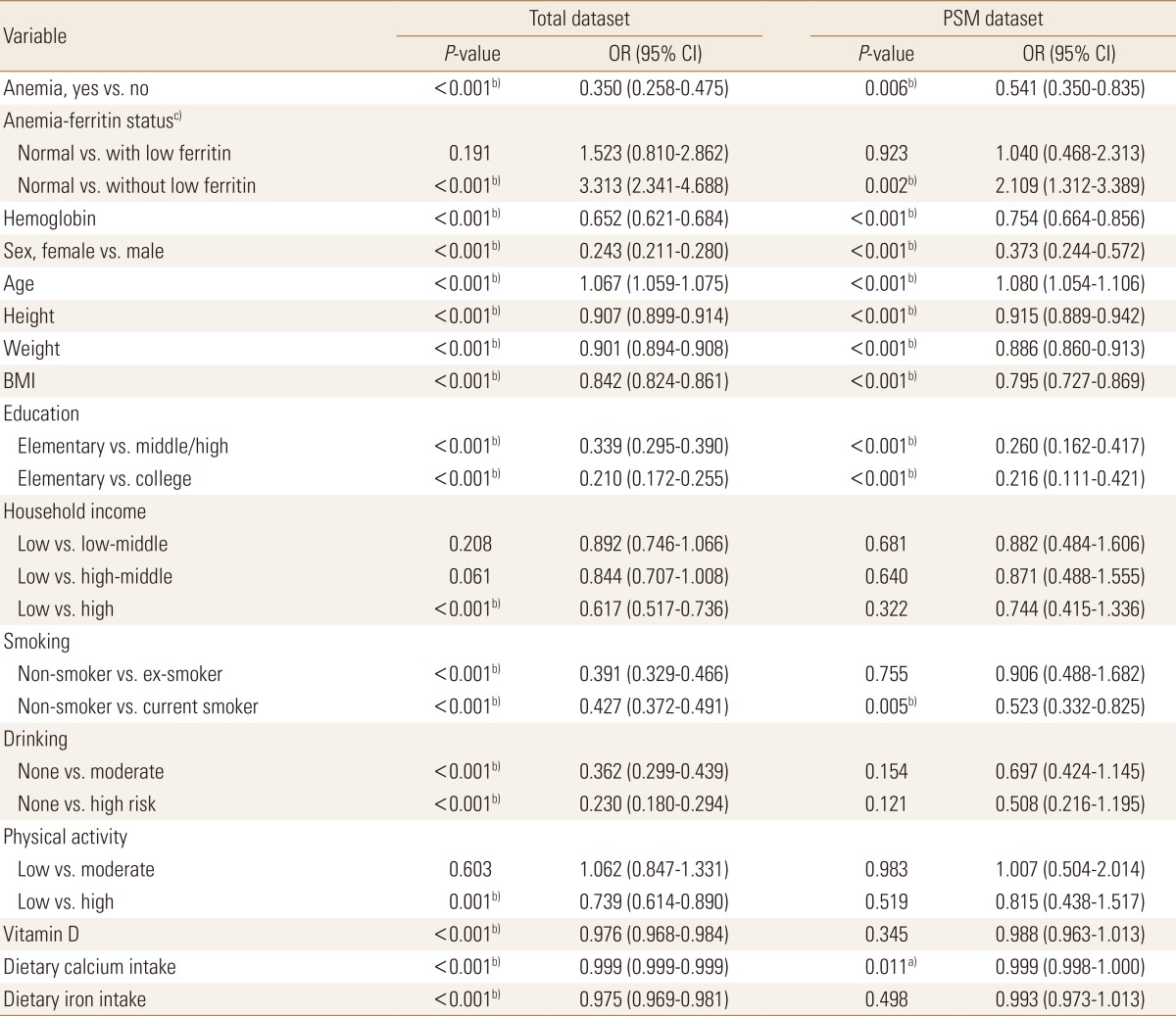
Comparison between total dataset and PSM dataset on logistic regression result (anemia, anemia-ferritin status effect for osteopenia or osteoporosis)
Second, a regression analysis was performed to determine the linear relationship between Hb levels and BMD scores at each site (whole body, femoral neck, and lumbar spine) in the subjects selected using the PSM method as the main analysis group. The regression analysis was performed after adjusting for variables such as age, BMI, drinking frequency, educational level, and physical activity.[2425] To examine the effects of age, the BMD at each site was divided into three age groups (20-49, 50-59, and 60 years and older) and analyzed. In addition, to examine the effects of menopause in women, the female subjects were divided into a premenopausal group and a postmenopausal group, and their data were analyzed. The variance inflation factor (VIF) was calculated to check the multicollinearity between the variables included in the multivariate analysis.
Third, the subjects were divided into two groups (the anemia and normal groups) and then into three groups (normal Hb, anemia with low ferritin, and anemia without low ferritin groups) according to Hb and serum ferritin levels. The difference in BMD score at each site between the groups was tested. Differences in BMD between the two groups according to the presence or absence of anemia were analyzed using an independent two-samples t-test, and differences in BMD among the three groups according to anemia-ferritin levels were tested using ANOVA. When differences between the groups were found to be significant in the analysis of variance, Scheffe's method was applied as a multiple comparison method.
Data from the KNHNES were obtained using a stratified, multi-staged, clustered, and probability sampling method with a composite sample design to improve the representativeness and accuracy of the samples. Therefore, the process of assigning weights, stratification variables, and cluster variables was included in the analyses in the present study. In addition, in the integration of the data from the KNHNES from 2008 to 2011, which included BMD measurements, integrated weights were calculated and applied in accordance with the “Guidelines for the use of raw data from the KNHNES”.
4. Study ethical considerations
The present study used data from the fourth and fifth KNHNES (2008-2011), which was approved by the institutional review board at Korea Centers for Disease Control and Prevention (Approval Nos. 2008-04 EXP-01-C, 2009-01CON-03-2C, 2010-02CON-21-C, and 2011-02CON-06-C). The data were downloaded from the respective public website. The present study was conducted in compliance with the ethical standards of the Declaration of Helsinki.
RESULTS
1. General characteristics of the subjects
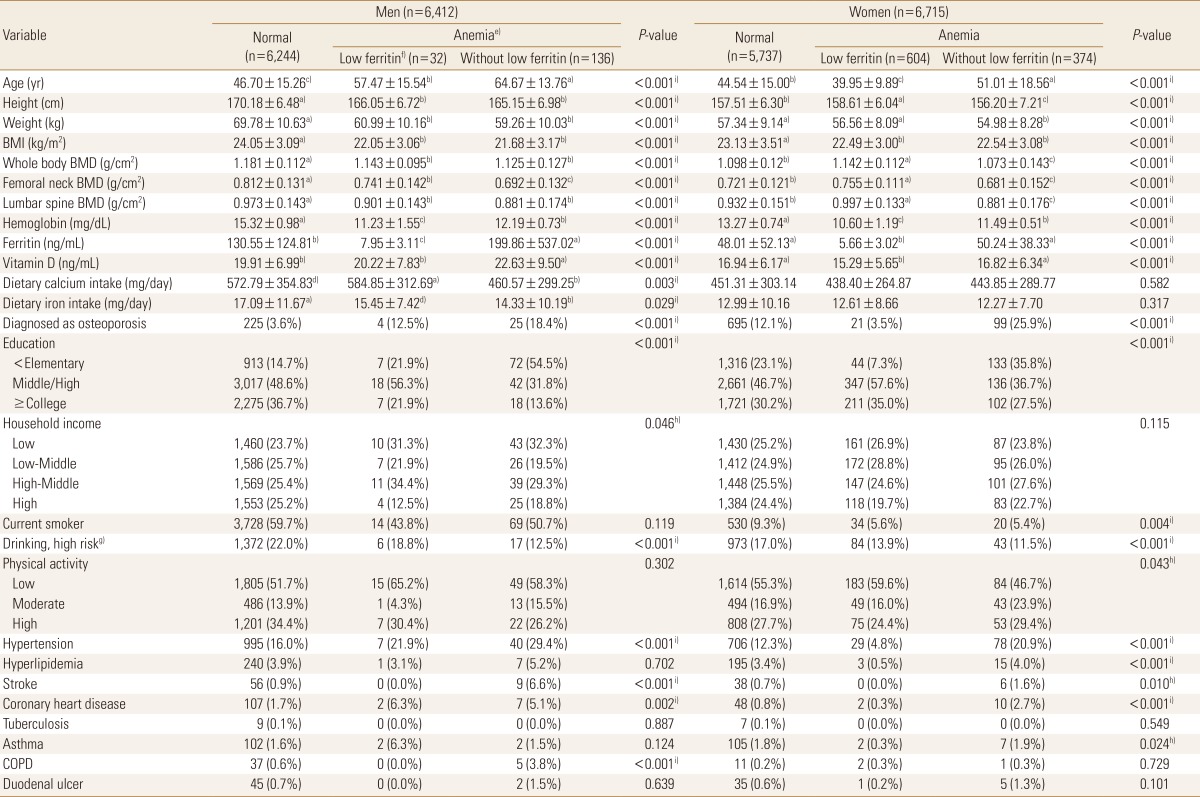
A total of 13,127 men and women were included in the original dataset before the PSM method was applied, and the baseline information is shown in Table 1. When the subjects were divided into the normal, anemia with low ferritin, and anemia without low ferritin groups, and the characteristics of each group were compared between the groups, the results showed significant differences in most variables between the groups.
The mean age of male subjects was highest at 64.67±13.76 years in the anemia without low ferritin group, followed by 57.47±15.54 years in the anemia with low ferritin group and 46.70±15.26 years in the normal group. The mean age of the female subjects was highest at 51.01±18.56 years in the anemia without low ferritin group, followed by 44.54±15.00 years in the normal group and 39.95±9.89 years in the anemia with low ferritin group.
In the men, BMI was highest in the normal group (24.05±3.09 kg/m2), followed by the anemia with low ferritin group (22.05±3.06 kg/m2) and the anemia without low ferritin group (21.68±3.17 kg/m2). Meanwhile, in the women, BMI was highest in the normal group (23.13±3.51 kg/m2), followed by the anemia with low ferritin group (22.49±3.00 kg/m2) and anemia without low ferritin group (22.54±3.08 kg/m2).
In terms of educational level, the proportion of elementary school graduates or lower in men was highest in the anemia without low ferritin group (54.5%), and this was the same for women (35.8%).
In men, the mean BMD (whole body, femoral neck, and lumbar spine) was highest in the normal group, followed by the anemia with low ferritin and anemia without low ferritin groups. In the women, the mean BMD was highest in the anemia with low ferritin group, followed by the normal and anemia without low ferritin groups.
When comorbid disease status was compared between the groups, the results showed that in the men, the prevalence rates of hypertension (29.4%), stroke (6.6%), and chronic obstructive pulmonary disease (3.8%) were relatively high in the anemia without low ferritin group, which had the lowest mean BMD among the three groups. In the women, the prevalence rates of hypertension (20.9%), stroke (1.6%), and coronary heart disease (2.7%) were relatively high in the anemia without low ferritin group, which had the lowest mean BMD.
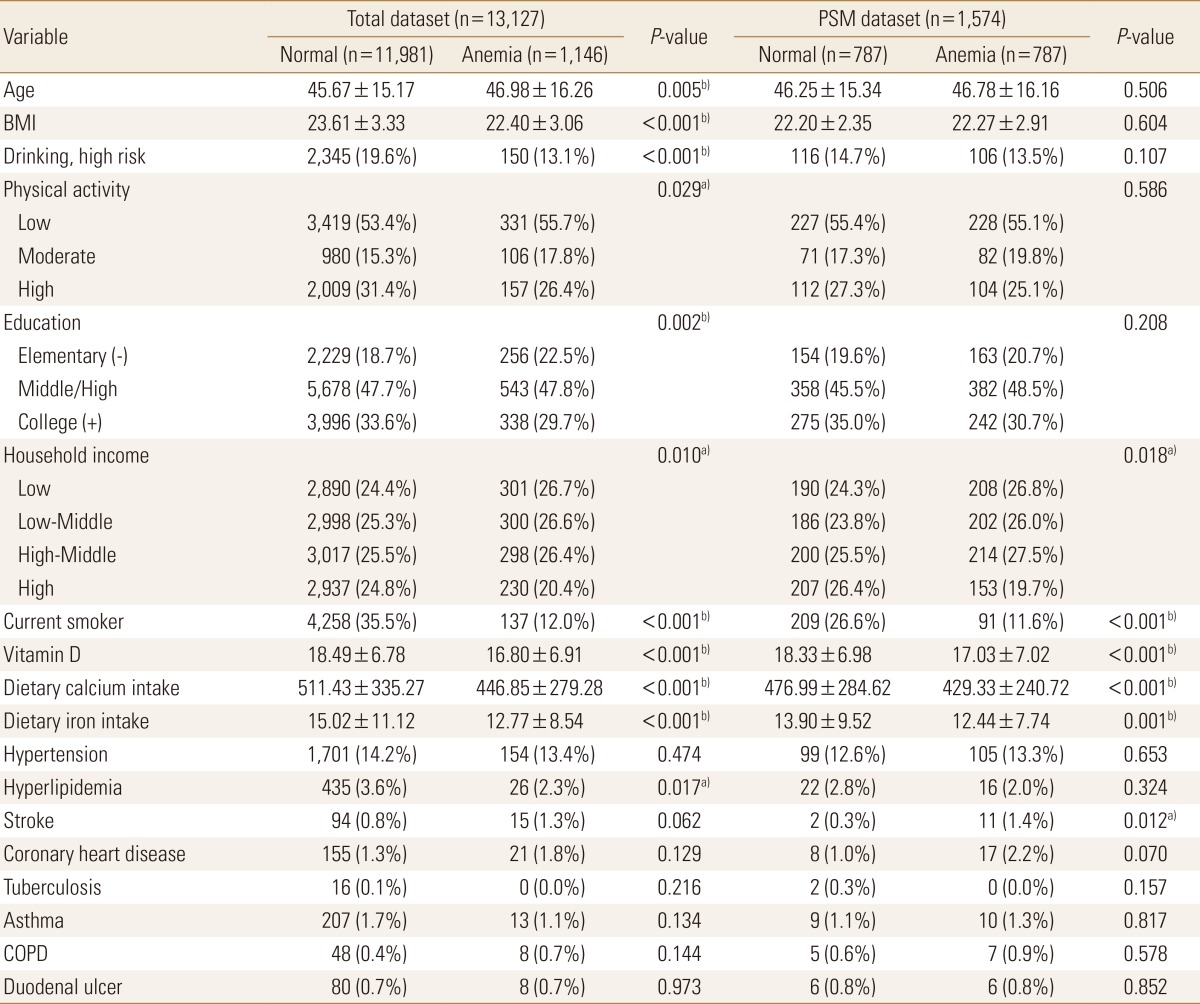
A comparison of the characteristics of the original dataset with those of the PSM dataset is shown in Table 2. The PSM dataset showed no difference in age, BMI, educational level, drinking frequency, and physical activity, which were selected as covariates between the normal and anemia groups. In addition, with regard to the prevalence of comorbid diseases, the PSM dataset showed a difference only in the prevalence of stroke between the normal and anemia groups (P=0.012), but showed no significant differences in the prevalence of hypertension, hyperlipidemia, ischemic heart disease, tuberculosis, asthma, chronic obstructive pulmonary disease, and duodenal ulcer between the normal and anemia groups.
To check the selection bias of the PSM dataset, logistic regression analysis with the osteopenic bone diseases (osteoporosis or osteopenia) as dependent variables was performed both with the original dataset and PSM data (Table 3). The OR from the anemia group to the normal group in the original dataset was 0.350 (P<0.05), whereas the relevant OR in the PSM dataset was 0.541 (P<0.05), which were all statistically significant, and the direction of the ORs was also consistent. This was the same in comparing among the three groups according to the presence or absence of anemia and low ferritin level. The OR from the anemia with low ferritin group to the normal group in the original dataset was 1.523 (P=0.191), whereas the OR in the PSM dataset was 1.040 (P=0.923). The OR from the anemia without low ferritin group to the normal group in the original dataset was 3.313 (P<0.05), while the OR in the PSM dataset was 2.109 (P<0.05), showing the same results in terms of both statistical significance and direction of ORs.
2. Analysis of the linear association between Hb levels and BMD at each site
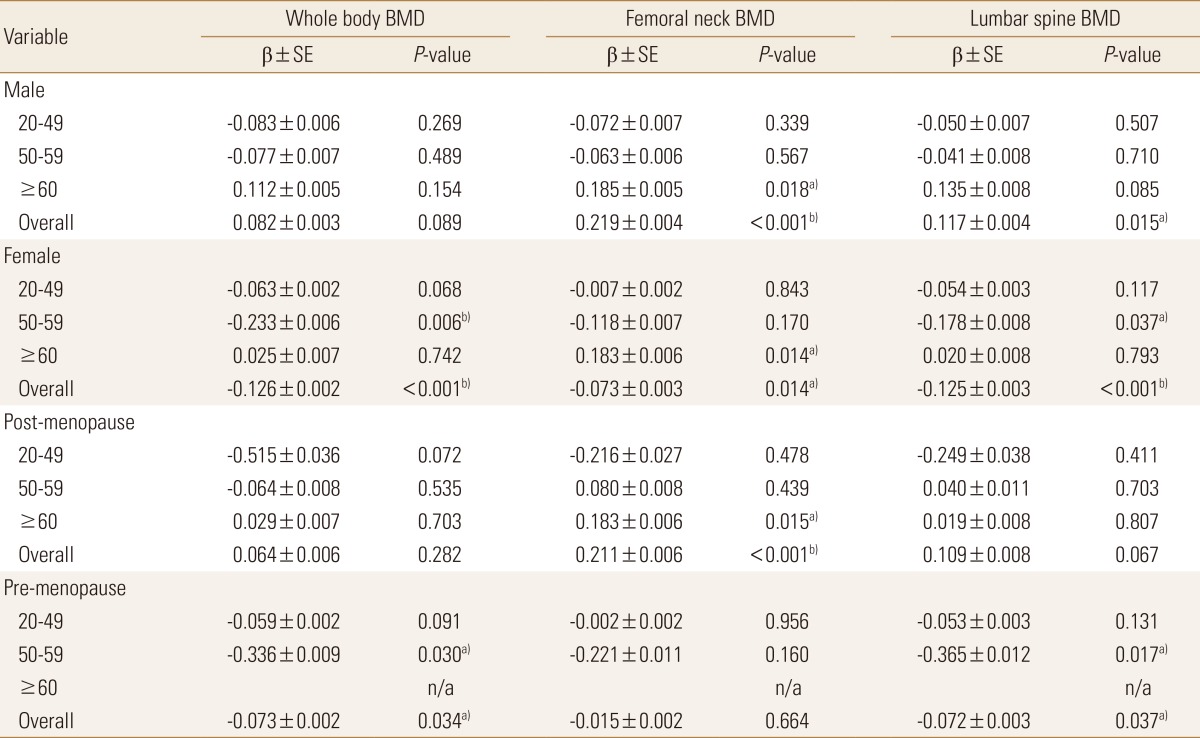
In the analysis of the linear relationship between Hb level and BMD at each site, the result showed significant positive associations between Hb level and femoral neck BMD (β=0.219, P<0.01) and lumbar spine BMD (β=0.117, P= 0.015) for men in all ages but significant negative associations between Hb level and whole-body BMD (β=−0.126, P<0.01), femoral neck BMD (β=−0.073, P=0.014), and lumbar spine BMD (β=−0.125, P<0.01) in the women. However, in the analysis by age group, the results showed a significant positive association between Hb level and femoral neck BMD (β=0.183, P<0.05) in the men aged ≥60 years, but no statistically significant association between Hb level and BMD at other sites in the other age groups. In the women, significant negative associations were found between Hb level and whole-body BMD (β=−0.233, P=0.006) and lumbar spine BMD (β=−0.178, P=0.037) in those aged 50 to 59 years, and between Hb level and femoral neck BMD (β=−0.073, P=0.014) in those aged ≥60 years.
In the comparison between premenopausal and menopausal women, negative associations were found between Hb level and whole-body BMD (β=−0.073, P=0.034) and lumbar spine BMD (β=−0.072, P=0.037) in the premenopausal women, whereas a positive association was found between Hb level and femoral neck BMD (β=0.211, P<0.01) in the menopausal women (Table 4).
3. Analysis of mean BMD at each site according to anemia
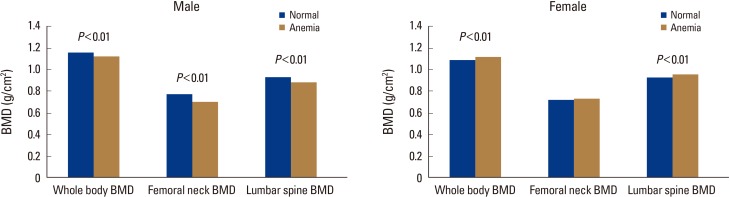
In the men, significant differences were observed in whole-body BMD, femoral neck BMD, and lumbar spine BMD according to the presence or absence of anemia. The normal group had significantly higher mean whole-body, femoral neck, and lumbar spine BMDs than the anemia group (P<0.01).
In the women as opposed to the men, the anemia group had higher whole-body and lumbar spine BMD scores than the normal group, showing statistically significant differences (P<0.01) (Fig. 2).
4. Analysis of differences in mean BMD according to anemia and serum ferritin level among the groups
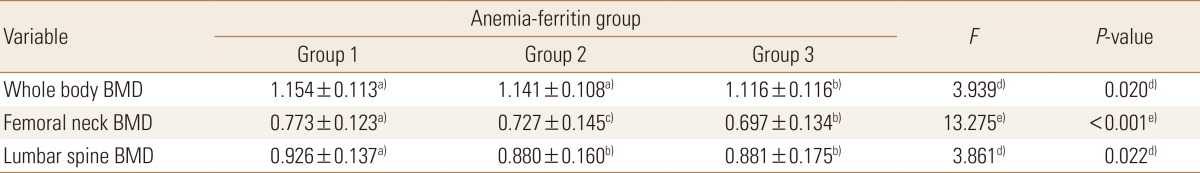
The men and women were found to have an opposite BMD pattern according to the presence or absence of anemia; thus, an additional analysis was performed. Statistically significant differences in whole-body, femoral neck, and lumbar spine BMD were found among the three groups of men. Whole-body BMD was significantly lower in the anemia without low ferritin group (1.116±0.116 g/cm2) than in the normal or anemia with low ferritin group (P=0.020). Femoral neck BMD was significantly higher in the normal group (0.773±0.123 g/cm2) than in the anemia without low ferritin group (0.697±0.134 g/cm2; P<0.001). Lumbar spine BMD was significantly higher in the normal group (0.926±0.137 g/cm2) than in the anemia with low ferritin group (0.880±0.160 g/cm2) or the anemia without low ferritin group (0.881±0.175 g/cm2; P=0.022) (Table 5A).
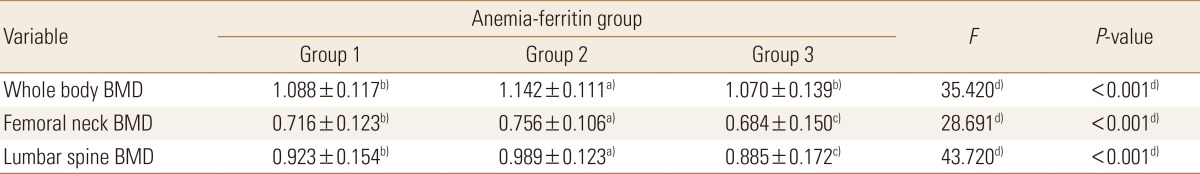
Meanwhile, significant differences were found in whole-body, femoral neck, and lumbar spine BMDs in the women. However, the BMD scores at all sites were highest in the anemia with low ferritin group, showing a difference from those in the men. Whole-body BMD was significantly higher in the anemia with low ferritin group (1.142±0.111 g/cm2) than in the normal group (1.088±0.117 g/cm2) or the anemia without low ferritin group (1.070±0.139 g/cm2; P<0.001). Significant differences in femoral neck BMD were found in the order of the anemia with low ferritin group (0.756±0.106 g/cm2) > the normal group (0.716±0.123 g/cm2) > the anemia without low ferritin group (0.684±0.150 g/cm2; P<0.001). Significant differences were also found in lumbar spine BMD in the order of the anemia with low ferritin group (0.989±0.123 g/cm2) > the normal group (0.923±0.154 g/cm2) > the anemia without low ferritin group (0.885±0.172 g/cm2; P<0.001) (Table 5A, B, Fig. 3).
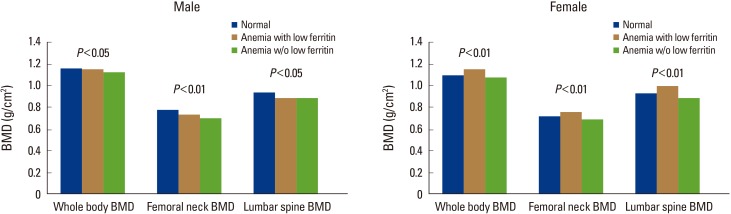
Bone mineral densities (BMDs) of whole body, femoral neck, and lumbar spine across anemia-serum ferritin categories by sex. w/o, without.
We performed a subgroup analysis according to menopausal status in the women. In the premenopausal women, only the mean whole-body BMD was significantly higher in the anemia with low ferritin group (1.147±0.105 g/cm2) than in the normal group (1.125±0.098 g/cm2) or the anemia without low ferritin group (1.141±0.106 g/cm2; P=0.022). In the menopausal women, significant differences were found in whole-body, femoral neck, and lumbar spine BMDs. Whole-body BMD was higher in the anemia with low ferritin group (1.048±0.175 g/cm2) than in the normal group (1.010±0.114 g/cm2) or the anemia without low ferritin group (0.983±0.125 g/cm2; P=0.049). Femoral neck BMD was significantly higher in the normal group (0.628±0.117 g/cm2) than in the anemia without low ferritin group (0.574±0.119 g/cm2; P=0.001). No significant difference in lumbar spine BMD was found between the normal group (0.813±0.158 g/cm2) and the anemia with low ferritin group (0.823±0.161 g/cm2), but lumbar spine BMD was significantly lower in the anemia without low ferritin group (0.766±0.145 g/cm2) than in the other two groups (P=0.035). In other words, a significant difference was found only in mean whole-body BMD between the groups of premenopausal women, whereas the BMD scores at all three sites were markedly low in the anemia without low ferritin group of menopausal women (Table 6).
DISCUSSION
In the present study, the association between Hb level and BMD was analyzed using various methods. According to the results of previous studies,[8910] chronic hypoxia is likely to interfere with bone metabolism. Increased oxidative stress and extracellular acidification under hypoxic conditions are estimated to influence bone formation and remodeling. In addition, previous studies suggested the association of whole body BMD loss and osteoporosis with various diseases such as inflammatory arthritis, inflammatory bowel disease, celiac disease, asthma, or chronic obstructive pulmonary disease, which cause a chronic inflammatory state of the body.[26] A specific study indicated that proinflammatory cytokines affect the development of anemia and osteoporosis.[27] In addition, osteoporotic tendency is observed in diseases that continuously affect hematopoietic function, such as sickle cell anemia,[11] thalassemia,[28] and Fanconi anemia.[29] Although no clear mechanism of such an osteoporotic tendency has been established, a hypothesis explains BMD loss on the basis of hematological etiology.[16] According to the hypothesis, blood loss stimulates the proliferation of hematopoietic progenitor cells and increases the number of hematopoietic cells, including hematopoietic growth factors and osteoclasts. Increased number of osteoclasts intensifies the resorption of bone tissue. In addition, reduced blood volume due to blood loss stimulates bone development and results in increased number of osteoblasts, thereby promoting new bone formation. As bone formation is promoted, it again increases the production of osteoclasts, resulting in increased bone resorption with subsequent increased hematopoietic area in the bone. The main point of the hypothesis is that if the above-mentioned process is repeated dozens of times, such as women's menstruations, the formation potential of osteogenic cells, including osteoblasts, becomes fatigued and if the process is repeated over a long period, it may result in BMD loss. However, considering that anemia is a phenotype of various physical or disease states, we can assume that the pathogenesis of BMD loss may be differ depending on the etiology of anemia.
The results of the present study showed that in the men, the BMD in the anemia group was lower than that in the normal group, whereas in women, the BMD in the anemia group was higher than that in the normal group (Fig. 2). In addition, when the anemia group was divided into groups according to the presence or absence of low ferritin level to compare between groups, the results showed that in the men, the mean BMD was highest in the normal group and lowest in the anemia without low ferritin group, whereas in the women, the mean BMD was highest in the anemia with low ferritin group and lowest in the anemia without low ferritin group as found in the men (Table 5A, B, Fig. 3). When the BMD in premenopausal and menopausal women was divided into three groups and compared, the results showed marked BMD loss in the anemia without low ferritin group among the menopausal women (Table 6).
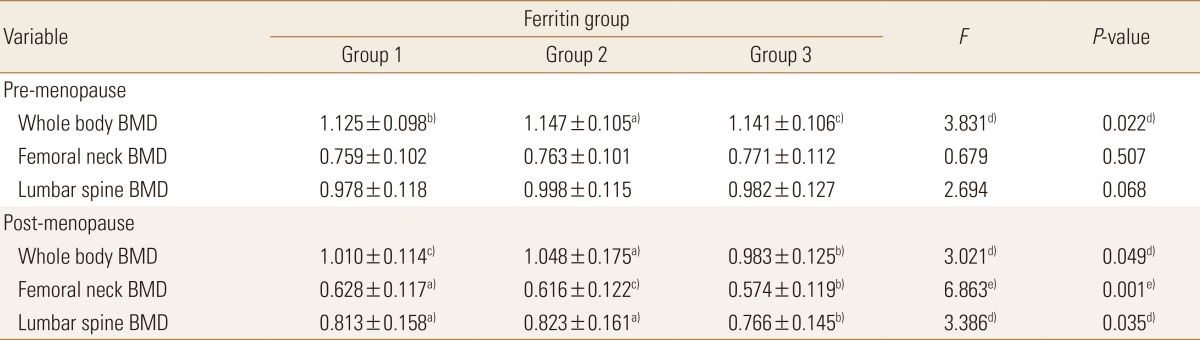
BMDs of whole body, femoral neck, and lumbar spine across anemia-serum ferritin categories and menopausal status
The differences in results between the men and the women in terms of BMD according to the presence or absence of anemia suggest that they may be due to the pathological difference between anemia in men and that in women. The fact that in the men, women, and menopausal women, the BMD was found to be consistently lower in the anemia without low ferritin group than in the normal or anemia with low ferritin group suggests that anemia, which is manifested as a phenotype of various diseases such as chronic inflammation or diseases with hypoxic condition but not due to blood loss or lack of iron intake, may be accompanied by BMD loss. In the present study, various diseases (chronic renal failure, liver cirrhosis, diabetes, thyroid disease, rheumatoid arthritis, and all types of cancer) that are known to interfere with bone metabolism were excluded. However, we compared the prevalence rates of diseases, which are expected to cause chronic inflammation and hypoxia in addition to the aforementioned diseases, among the three groups. As a result, diseases with remarkably high prevalence rates in the anemia without low ferritin group included hypertension (29.9%), stroke (6.7%), and chronic obstructive pulmonary disease (3.8%) in men, and hypertension (20.9%), stroke (1.6%), and coronary artery disease (2.7%) in the women. However, because the prevalence of other diseases except for hypertension was not high enough to account for all the causes of anemia without low ferritin level, the subjects with anemia without low ferritin level were likely to have a disease that was not surveyed during the KNHNES data collection or to be unaware of their disease.
The finding of the present study that the mean BMD was higher in the anemia group with low ferritin level than the other two groups of women was unusual. Anemia with low ferritin suggests a deficiency of iron in the body, and the anemia in this case is likely to be iron-deficiency anemia on the basis of the pathogenesis of the anemia. In general, the most common anemia in women is iron-deficiency anemia, and women of childbearing age who weighs approximately 60 kg without a specific underlying disease are known to consume approximately 10 mg more iron than any men or women who do not have menstruations.[30] However, iron-deficiency anemia in older persons is known to be due to a low intake of iron or lack of absorption rather than due to periodic blood loss, unlike iron-deficiency anemia in premenopausal women.[31] In the case of anemia caused by chronic inflammation or iron-deficiency anemia caused by a lack of iron intake or absorption, only mechanisms underlying hypoxic and inflammatory bone metabolism inhibition are present, without any factor that promotes bone metabolism. However, in the case of iron-deficiency anemia due to blood loss, a mechanism of bone formation stimulation exists due to the proliferation of osteoblasts, on the basis of the aforementioned hematological hypothesis. If the bone formation stimulation effect of blood loss is relatively larger than the bone absorption effect until the overall bone formation ability of osteogenic cells is lowered, the periodic blood loss may have a promoting effect on bone formation. The finding of the present study that the mean BMD was highest in the anemia with low ferritin group of women can be one of indirect evidence, and this was also partly suggested by the results of comparing BMD between premenopausal and menopausal women. This was also in line with the findings that in premenopausal women, whole-body BMD was significantly higher in the anemia with low ferritin group than in the other two groups and the lumbar spine BMD was not statistically significant but was highest in the anemia with low ferritin group among the three groups. On the other hand, the present study found that in the menopausal women, bone loss was noticeable in the anemia without low ferritin group, but the BMD was not higher in the anemia with low ferritin group than in the other two groups. Although the aforementioned results of the present study cannot be said to be a direct evidence for supporting the hypothesis of bone metabolism based on hematological etiology, the results can be explained to some extent by the mechanism of the hypothesis.
The results of the present study suggest that reduced Hb levels or anemia findings do not necessarily mean BMD loss, but patients with anemia as a phenotype of various diseases other than typical iron-deficiency anemia are highly likely to also have BMD loss. Therefore, evaluation of BMD in patients with underlying disease and anemia findings may be meaningful.
The present study has limitations. This was not a prospective study but was a cross-sectional study, so it cannot explain the causal relationship between BMD and Hb levels, and the available variable for determining the pathogenesis of anemia was limited to serum ferritin levels. If the iron-binding ability or general blood test data from the fifth and succeeding KNHNES were included for analysis, we could classify the subtypes of anemia and analyze more accurately the association between the subtypes of anemia and BMD. In addition, because the number of diseases investigated in the present study was limited, it is regrettable that there was a limitation in the identification of comorbid diseases in the anemia without low ferritin group or the underlying causes of anemia with low ferritin level, which is uncommon in men. Finally, the number of men with anemia was small, with only approximately one sixth of those with anemia in women.
Future studies regarding the association between Hb levels and BMD use markers that reflect bone formation (like osteocalcin, bone specific alkaline phosphatase) and bone resorption (N-telopeptide of collagen cross-link, C-telopeptide of collagen cross-link, deoxypyridinoline, etc.) in subjects for study designs that can verify hypotheses based on related hematological etiology [32] and long-term follow-up studies of the mechanism of anemia will be helpful to clearly understand the association between Hb levels and BMD.