Serum Bisphenol A Concentration in Postmenopausal Women with Osteoporosis
Article information
Abstract
Objectives
Bishphenol A (BPA) is a representative endocrine disruptor and is also known as a xenoestrogen. The objective of the present study is to investigate how many patients are exposed to BPA and to analyze the relationships between serum BPA concentration, bone mineral density (BMD) and biochemical bone markers in postmenopausal women with osteoporosis.
Methods
Total 51 patients were enrolled for measuring BPA and clinical variables including BMD and bone markers. The relationship between BPA and clinical variables were analyzed by the Pearson's correlation test and the Kruskal-Wallis test. Serum BPA concentration was measured by enzyme linked immunosorbent assay (ELISA).
Results
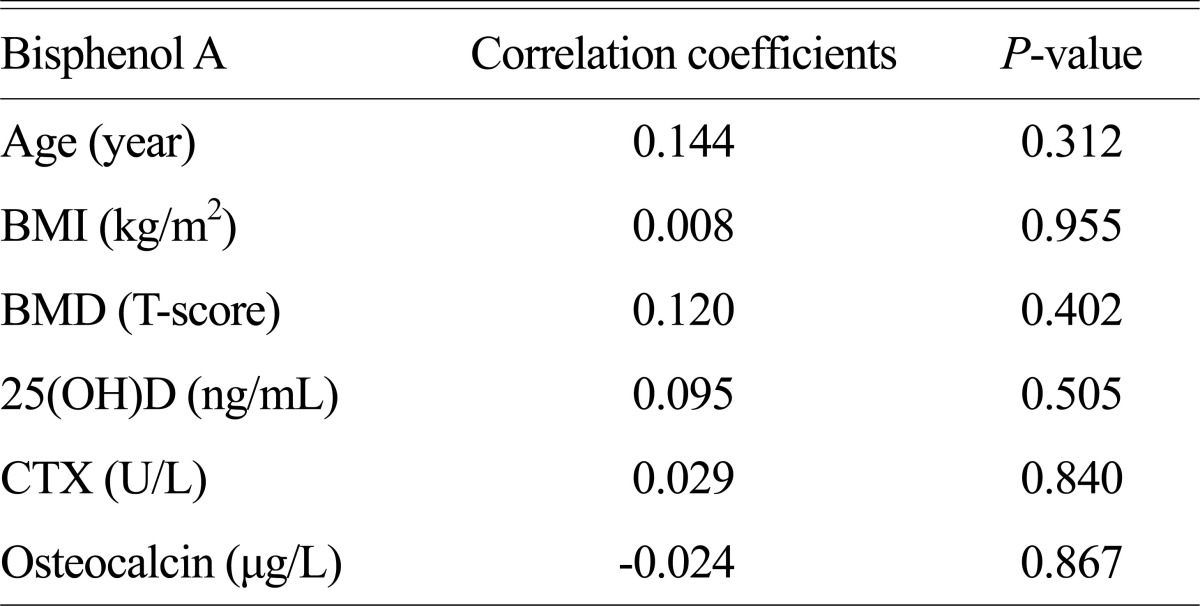
BPA was detected in all samples. The mean BPA concentration was 1.44 ± 0.52 ng/mL. There was no statistically significant correlation between BPA and clinical variables.
Conclusion
There was no statistical significance between serum BPA concentration and clinical variables related to bone metabolism. To clarify the effect of BPA on bone metabolism, further large scaled and high risk group investigation may be needed.
INTRODUCTION
Bisphenol A (BPA) is well known as a representative endocrine disruptor interrupting body functions controlled by hormones.[1] Approximately 2.2 million tons of BPA are produced annually all around the world and widely applied in the manufacture of an epoxy resin, mainly used to produce a container or a package for foods and beverages, or a polycarbonate plastic.[2] BPA is widely and continuously exposed to human through a diet and is also exposed by the other paths such as through skin and dust etc. In fact, according to a research performed in the US, measureable BPA was found in approximately 93-95% of the subjects through urine.[3,4]
The mechanism that BPA shows inappropriate effect in the body is known as estrogen like mechanism;[5] besides, a decrease of the function in a pancreatic beta cell,[6] an interruption of the function in a thyroid hormone,[7] an obesity acceleration effect,[8] and the induction of an oxidative stress and an inflammation[9] are included. According to recent studies, the relationship with endocrine related diseases, including diabetes, cardiovascular diseases, liver diseases, and semen abnormalities have brought up constantly.[10,11]
Osteoporosis is a skeletal disease occurred in postmenopausal women and elderly and increases the risk of a bone fracture due to a bone mass reduction and microstructure deterioration.[12] Of the known mechanisms up to date, an estrogen deficiency is the most important factor in the osteoporosis occurrence.[13]
Considering the relationship of an estrogen deficiency and an osteoporosis occurrence, a xenoestrogen is also thought to be related to the occurrence of osteoporosis; but until today some studies investigating the relationship of BPA, a xenoestrogen, and bone metabolism have only performed in in vitro or utilizing animals. Suzuki et al.[14] reported that tartrate-resistant acid phosphatase (TRAP) and alkaline phosphatase (ALP), the biochemical bone marker, decreased significantly when injecting BPA in in vitro experiment using goldfish scales. Also Seidlová-Wuttke et al.[15] examined that bone-forming factors increased and bone-resorbing factors decreased in the rat group that a higher volume of BPA was injected but a bone mineral density (BMD) decreased. However there was no study investigating the relationship of a blood BPA concentration and osteoporosis in human until today. The objectives of the study were to examine how many patients were exposed to BPA by measuring serum BPA in the patients with osteoporosis and to analyze the relationships with a BMD and a biochemical bone marker.
SUBJECTS AND METHODS
1. Subjects
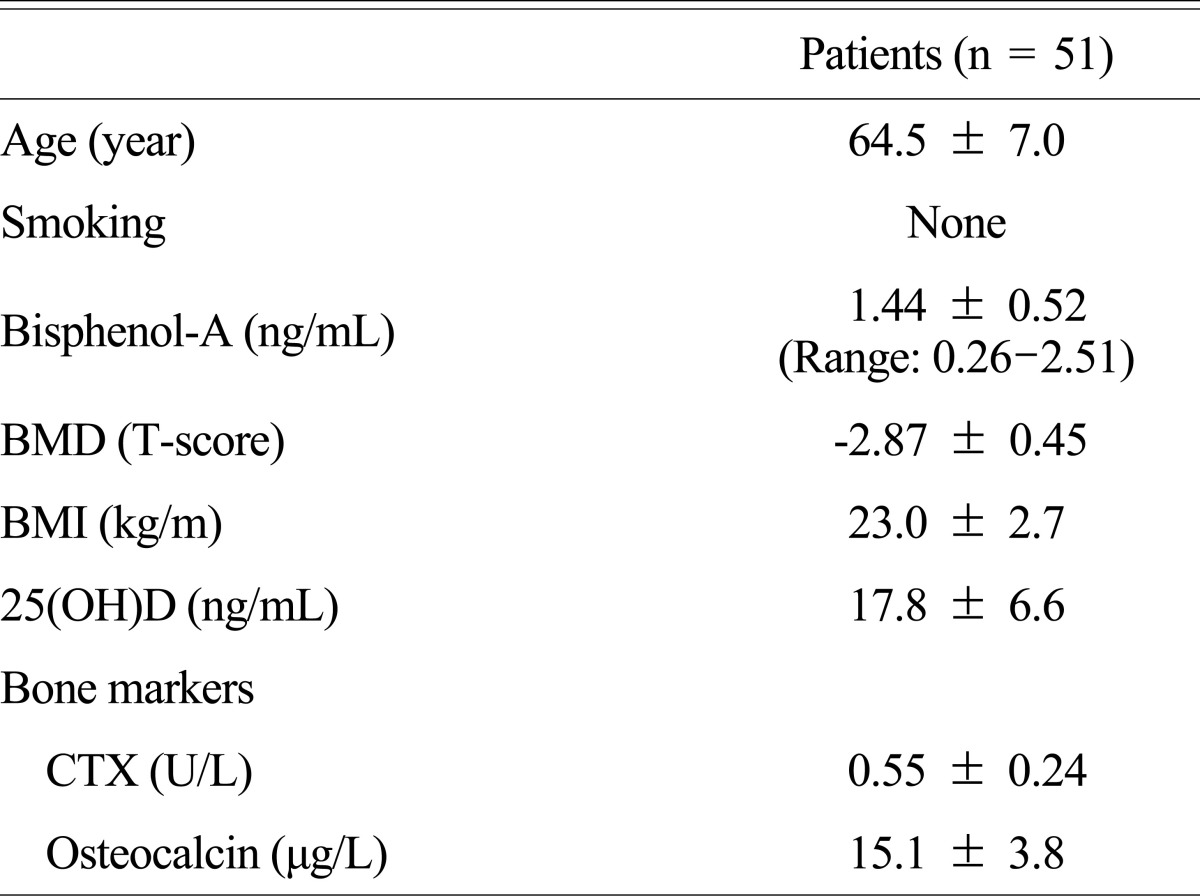
In order to examine the relationship between serum BPA concentration and a BMD and a bone marker, the study was carried out in 51 postmenopausal women who had a treatment due to osteoporosis from October 2008 to July 2009 in Kyung Hee University Hospital at Gangdong; all patients were lived in Seoul or Hanam-si. Of the postmenopausal women who are more than 50 years old, the subjects were in the case where T-score of an average lumbar (L1-4), a femoral neck, or the total of BMD was less than -2.5 or T-score was less than -1.0 with at least more than a radiologic finding in a spine fracture. Menopause was defined in case where there was no natural menstruation more than a year at least or follicle stimulating hormone (FSH) exceeds 40 IU/L in case of the subjects with hysterectomy. There was no patient taking medications affecting bone or calcium metabolism or having a malignant tumor or other systemic diseases. The present study was performed after the review of the organization review committee and a written consent form was received from all the participants.
2. Methods
Body mass index (BMI), blood 25-hydroxyvitamin D (25[OH]D; DiaSorin Corporation, Stillwater, MN, USA), C-telopeptide of collagen cross-links (CTX; Roche Diagnostics, Mannheim, Germany), and osteocalcin (OC; CIS Bio International, Gif-sur-Yvette, France) of the subjects were investigated at the time of measuring the BMD. The BMD was measured in the lumber number 1 through 4, a total femur, and a femoral neck using dual energy X-ray absorptiometry (DXA; Lunar Prodigy Advance, GE Lunar, Madison, WI, USA) and was expressed as g/cm2.
In the study, a pre-processing was carried out in order to measure BPA concentration of the serum separated from blood in the subjects. The samples were passed through a SPE column (Isolute M-M cartridge) in order to remove high molecular proteins and lipid components in the serum and concentrate the samples. Then the samples were washed with 200 µL of 10% methanol. An extraction was carried out using 200 µL of an extraction solution (methanol: acetonitrile, 3:1). Once evaporated, the samples were reconstituted in 40 µL of analysis buffer (0.01 M phosphate buffer, pH 7.4).
After such procedures, the blood BPA concentration was measured utilizing Ecologiena BPA enzyme linked immunosorbent assay (ELISA) Kit (Japan Environchemicals, Tokyo, Japan). The measurement method was developed by utilizing the characteristic that a monoclonal antibody bound to BPA only and didn't bind to any other chemical substances with a similar structure. A quantitative analysis range was 0.05-10 µg/L (ppb) and sensitivity was good enough to measure BPA present in the serum. The results were shown a high reproducibility and coefficient variation (CV) was less than 10% in general.
3. Statistical analysis
Results were expressed in an average and a standard deviation and significance of the relationship between clinical factors including blood BPA concentration of the experimental group, a BMD, and a biochemical bone marker etc were verified utilizing the Pearson's correlation test. Additionally, partial correlation analysis was used to control the confounding variables such as age and BMI that could have an influence on the blood BPA concentration. Also the subjects were classified into three groups based upon several clinical factors and then Kruskal-Wallis test was used in order to compare average BPA concentration of each group. It was considered statistically significant when P is less than 0.05 in all cases. Statistical analysis was performed utilizing SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
The average age of 51 subjects participated in the study was 64.5 (50-82 years old) and all of them were nonsmokers. The subjects showed the pattern of overweight with BMI 23 and the BMD T-score was -2.87. Average 25(OH)D concentration was 17.8 ng/mL (Table 1).
All subjects showed BPA of more than measureable concentration. The average concentration of the blood BPA was 1.44 ng/mL and didn't show statistically significant relationship with the BMD. Also 25(OH)D, CTX, and OC measured as clinical variables related to bone metabolism had no statistically significant relationship with the blood BPA concentration (Table 2). The results were examined in the same manner when correcting based upon age and BMI.
Since few subjects were participated in the study, the clinical variables including a BMD and the concentration of a biochemical bone marker etc were classified into three groups (average - under 1 standard deviation [SD], average ± 1SD, average + 1SD excess group) based upon averages and then analyzed if there was a difference in blood BPA concentration. No significant difference was observed in the blood BPA concentration of all indexes among the groups (Table 3).
DISCUSSION
Environmental estrogens are parts of xenoestrogens and bind to estrogen receptors thereby exhibiting their functions. If organs with estrogen receptors are continuously exposed to such xenoestrogens, they may either act like inherent estrogens that are synthesized in the body or represent opposite effects as xenoestrogens inhibit the bindings between inherent estrogens and receptors. In the former cases, xenoestrogens act agonistically while they affect antagonistically in the later cases. Of the xenoestrogens, representative environmental estrogens include BPA and benzopyrene which are classified as endocrine disruptors.
A environmental estrogen, BPA which is a compound of interests in the study, shows various exposure routes. It is mainly detected from plastic products and widely used for baby bottles, bowls, food containers, and cans as well as the production of epoxyresin which is in the amalgam for the dental clinic.[16] Lately, the exposure of BPA via medical devices has been an issue; it is known that there are highly frequent exposures for the patients with hemodialysis as circulating blood contact the polycarbonate devices of hemodialysis and extract BPA when performing the dialysis. [17] Furthermore, according to Le et al.[18], if boiling water was added, there was a 55 fold increase in the BPA concentrations compared to that of room temperature. It is also known that repetitive usages of containers are correlated with increase of BPA concentrations.[19] These absorbed BPA in the body are biological active unconjugated form but quickly conjugated in the liver so that excreted via bile or urine; the half life of this compound is 5.3 hr which is relatively short.[20-22]
The fast excretion of BPA due to its short half life has been adopted for the scientific evidences of BPA safety, but β-glucuronidases in multiples organs, especially lung, liver, and kidneys of rats and placenta of human deconjugate BPA thereby excreting active BPA.[23] Furthermore, according to recent studies, BPA represents 3.3 of a coefficient of correlation which is lipophilic and exhibits approximately 3 times higher affinity compared to kidneys and muscles, meaning that there are higher chances of accumulation and it may continuously affect the body metabolism.[29]
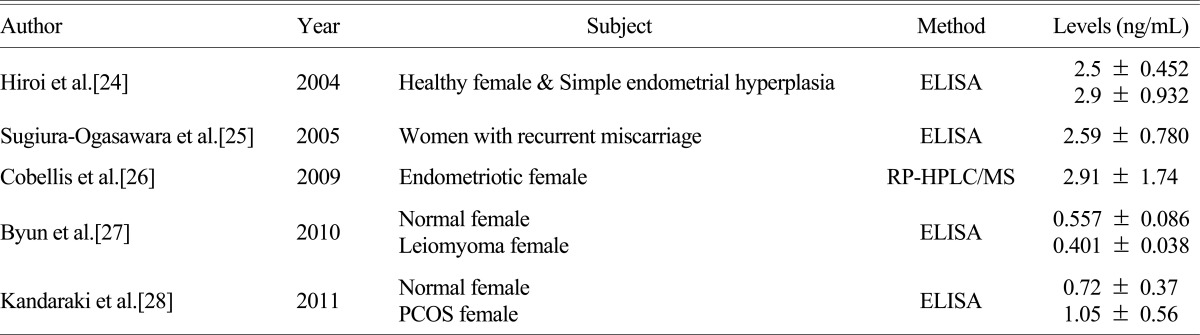
The concentrations of BPA in blood of postmenopausal women with osteoporosis were 1.44 ng/mL, which are significant. Compared to the values (0.437 ± 0.036 ng/mL, 83.9% detection rate) of population from the domestic study with uterine myoma patients,[27] these were relatively high levels. Such differences might because of higher exposure and accumulation of BPA in the body owing to relatively high ages and BMI of subjects as well as differences in residential areas. In addition, methods for BPA measurements, subjects, and results from domestic and foreign studies, are listed in Table 4. Depending upon the research subjects and various conditions, concentrations of BPA in the blood were different; yet it represented the significant association with female hormones related diseases including endometrial hyperplasia, habitual abortion, endometriosis, and polycystic ovary syndrome except for the domestic study with uterine myoma patients.
Likewise, the osteoporosis is one of the representative female hormones related diseases. So far, however there are only limited numbers of in vivo studies have been done with regard to the association between bone metabolism and BPA, a xenoestrogen. This study is the first study investigated the association between the blood BPA concentration and bone metabolism. Considering the effects of BPA, as a xenoestrogen, it was expected that there would either positive or negative correlation between the BPA level and biochemical markers that represent dynamic changes, but any significant correlation was not observed.
There were several limitations to draw significant results. First, there were a relatively small number of patient subjects. Second, although aging and deficits of female hormones are known risk factors for osteoporosis, they are only a part of multiple risk factors, meaning that this environmental estrogen, BPA would be just one of the many factors for such diseases; thus a consideration should be taken account regarding its limited effects on such diseases. Third, other than estrogen related one, the mode of action of BPA that affects the bone metabolism includes various mechanisms including the lipid metabolism as well as oxidative stress. Fourth, there is an issue in regards to the measurement of BPA concentration. That is, it would be necessary to understand better 1) how to measure the BPA 2) whether blood samples would reflect the accumulation of BPA in the body better than urine samples, or vice versa 3) if a single measurement of blood BPA would accurately show the accumulation level of BPA in the body. It is considered that there is a limitation to conclude about the causal relationship between the blood BPA concentration and the BMD as well as bone factors. Lastly, although we performed the partial correlation analysis for potential confounding variables (e.g., age, and BMI) regarding the blood BPA concentration, it might not be corrected enough and analyzed. Given the limitations, further additional large scale studies with various subject groups are warranted in order to clarify such relationships.
CONCLUSION
It has been emerged that the association between an endocrine disruptor such as BPA, a xenoestrogen, and various diseases. To the best of our knowledge, it was the first time to investigate the relationship between bone metabolism and BPA in human, but there were no significant correlations between the blood BPA concentration and a BMD as well as a biochemical bone marker. Further investigations are warranted for future.
Notes
This study was supported by KSBMR.