Coronary Calcification Is Reversely Related with Bone and Hair Calcium: The Relationship among Different Calcium Pools in Body
Article information
Abstract
Background
With aging, calcium efflux from bone is increased with age-related bone loss, and it can reduce bone mineral density (BMD). On the contrary, age-related calcium adoption into arterial wall progressively stiffens blood vessels. Theses process insinuates shift of calcium among different pools in body. However, their relationships have not been elucidated yet. So we investigated the correlation among calcium contents in different body pools, such as hair, bone, and blood vessels in women.
Methods
We analyzed 50 females retrospectively who measured Agatston coronary artery calcium score (CACS), BMD, and hair calcium concentration at a regular health check-up in a university hospital. CACS was achieved by coronary multidetector computed tomography, BMD was measured by dual energy X-ray absorptiometry in the lumbar spine and femur, and hair calcium level was checked by hair tissue mineral analysis.
Results
CACS inversely correlated with BMD (r=-0.280, P=0.049 with lumbar vertebrae 1-4, r=-0.310, P=0.028 with femur neck, r=-0.333, P=0.018 with femur total) and hair calcium concentration (r=-0.352, P=0.012).
Conclusions
CACS has negative correlation with BMD and hair calcium level in women. Different body calcium pools such as bone, hair and blood vessel significantly correlated each other.
INTRODUCTION
Calcium efflux from bone increases with aging as age-related bone resorption, and it can reduce bone mineral density (BMD).[1] This process resulting in osteoporosis and fragility fracture.[2] Whereas, increased calcium adoption in arterial wall, progressively stiffens blood vessels with aging,[3] leading to atherosclerosis and cardiovascular diseases (CVD).[4] Recent studies reported that BMD is negatively associated with the degree of calcification in abdominal aorta[56] and cardiovascular mortality.[7] These observations suggest the possibility that calcium migrated from bone and may settle down in vessel, and we can assume that there is shift of calcium among different calcium pools in body.
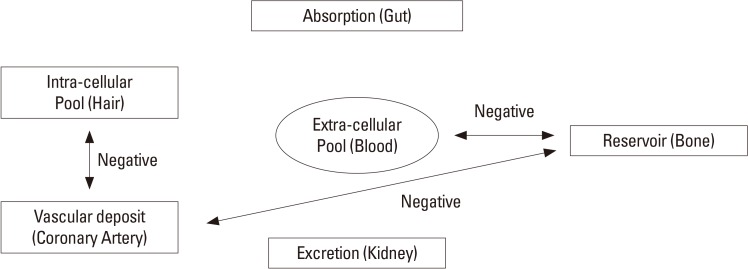
Calcium enters body from ingested food, passes through serum calcium pool, is distributed to intracellular pool and excreted through renal clearance. In this process, calcium also deposited in bone, the greatest reservoir of calcium, and other tissues including blood vessels (Fig. 1). Agatston et al.[8] successfully quantified the degree of calcification in coronary arterial wall, detected by multidetector computed tomography (MDCT) with accuracy and reliability.[8910] The coronary artery calcium score (CACS) is well correlated with extents of extracoronary atherosclerosis[11] and also known as a good predictor for CVD risk.[1213]
The hair calcium concentration can represent intracellular calcium level,[1415] so it has been reported as an indicator for deteriorations in bone metabolism[16] and as a predictor for coronary heart disease.[17] In addition, a recent research shows hair calcium level associated with calcium intake and BMD.[18] However, it has not well elucidated how one calcium pool interacts with others.
The authors assumed that all these calcium pools are closely connected. So, we investigated if calcium level of one calcium pool is correlated with those of other calcium pools in body to get a glimpse of how calcium mobilizes among different calcium pools in body with aging. So calcium contents in bone, serum, hair, and blood vessel were analyzed in our study.
METHODS
1. Study design and subjects
Current study is single center, retrospective observational study. We evaluated 74 females aged above 18 years, who performed CACS measurement, hair mineral analysis, BMD and calcium intake measurement at one medical checkup at Ajou University Health Promotion Center, Suwon, Korea from January 1st 2007 to June 30th 2011. Usually, major companies include these tests in their medical checkup program for board members or managers. If, there were more than two checkup data with these four tests during study period, only initial data were used. Exclusion criteria were three. First, individuals who have diseases or conditions that influence calcium metabolism, such as small intestine resection status, chronic renal failure and liver cirrhosis. Second, patients who have disease associated with bone mineralization, such as hyperparathyroidism, Cushing syndrome, adrenal insufficiency, hyperthyroidism, hypothyroidism, and rheumatoid arthritis. Third, participants who were taking any medications that can affect BMD, such as steroid hormones, thyroid hormones or anti-thyroid drugs, anticonvulsants, male and female hormone therapy including oral contraceptives, and calcium or vitamin D medications. Finally, 24 women excluded and 50 women were included. The Institutional Review Board of Ajou University Hospital in Suwon, South Korea, approved this study (AJIRB-MED-MDB-11-323).
2. Outcome measurement
CACS was represented by Agatston calcium score, which is suggested by Agatston et al.[8] at 1990. And, it is measured by 64-slice MDCT scanner (Brilliance 64; Philips Medical Systems, Best, the Netherlands). CACS was computed as the sum of all Hounsfield units (HU) in a lesion multiplied by the voxel volume in mm. Coronary artery calcification (CAC) was defined as a hyperattenuating focus (Four contiguous pixels, and was considered as tomographic numbers >130 HU for each pixel). A score for each focus of CAC was calculated by multiplying the focus area (mm2) by a density measurement defined by the peak CT number in the focus. The total CACS was calculated as the sum of scores for all foci in the epicardial arteries.
BMD was measured by using dual energy X-ray absorptiometry (DXA; Lunar Prodigy Advance, GE Lunar, Medison, WI, USA). Bone mineral content (BMC; g), area (cm2) and T-score were measured at the 1st, 2nd, 3rd, and 4th lumbar spine (L1-4), femoral neck and total femur. BMD (g/cm2) was calculated by dividing area (cm2) by BMC (g).
Hair samples (80 mg) were collected from four different points of occipital scalp. The samples were cut proximal portion of hair (3-4 cm from skin) with stainless steel sampling scissors. The participants were asked not to chemically process their hair for at least 2 weeks, such as dying, perm, straightening, or frosting. The hair also had to be free of all gels, oils, and hair creams before sample collection. Measurements were performed using a microwave temperature-controlled digestion technique and Perkin-Elmer Mass Spectrometer in a licensed and certified clinical laboratory that undergoes regular inspections with the Clinical Laboratory Division of the Department of Health and Human Services (Trace Elements Inc., Addison, TX, USA). The spectrophotometers of minerals were reported as unit of mg% (mg/100 g of hair).
Dietary calcium intake measured by 24-hr recalls method. The type and amount of food was recorded by direct interview with a nutritionist. Each mineral in daily diet was processed and evaluated using dietetic computer program, Computer Aided Nutritional analysis Program, version 3.0 (CAN-Pro; The Korean Nutrition Society, Seoul, Korea).
All participants underwent a full physical examination and anthropometric measurements. The anthropometric measurements were conducted with wearing light gown and no shoes, and after an overnight fasting. Height was measured to the nearest 0.1 cm, and weight was measured to the nearest 0.1 kg, by automatic height and weight measuring machine. Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2). Waist circumference was measured with a flexible tape, at the level of the umbilicus, and was recorded to the nearest millimeter. Systolic and diastolic blood pressures and pulse rates were measured by automatic blood pressure measuring machine at sitting position. Body fat percentage were measured at the fasting status, by Inbody 720 (Biospace Inc., Seoul, Korea), which is body composition estimator based on bioelectrical impedance analysis.
Blood samples were also collected after an overnight fasting. Measurements of serum glucose, total cholesterol, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, high-sensitivity C-reactive protein, homocysteine, calcium, blood urea nitrogen, and creatinine were performed.
3. Statistical analysis
All continuous variables were expressed as mean±standard deviation. One-sample Kolmogorov-Smirnov test was performed for normality test of all continuous variables. In normality test, CACS was non-normal distribution, and other major variables (hair and serum calcium level, dietary calcium intake and BMD) and age were normally distributed. Pearson's correlation test (in case of nonparametric variables, Spearman's correlation test) was performed for elucidating relationships between age and major variables, such as CACS, BMD, hair and serum calcium level and dietary calcium intake. All statistical analyses were two-tailed, and a P-value<0.05 was considered statistically significant. Data were analyzed by using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Baseline characteristics
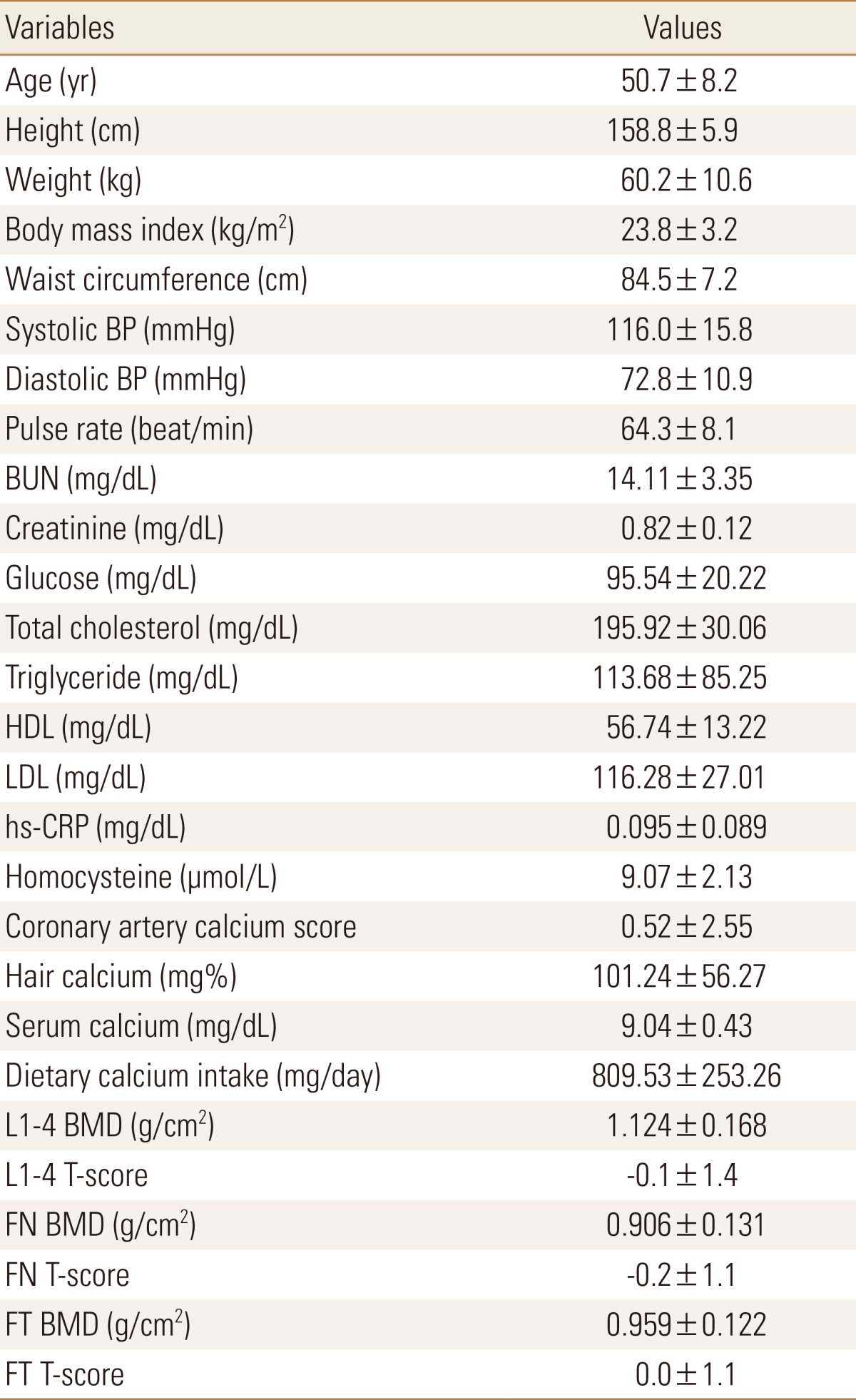
Baseline characteristics of study subjects were presented in Table 1. The mean age was 50.7±8.2 years (range, 40-75 years). The mean value of CACS was 0.52±2.55. Average hair calcium concentration was 101.24±56.27 mg%, average serum calcium level was 9.04±0.43 mg/dL, average serum creatinine concentration was 0.82±0.12 mg/dL, and average calcium intake was 809.53±253.26 mg/day. The mean BMD and T-score of L1-4 vertebrae were 1.124±0.168 g/cm2 and -0.1±1.4, and these of total femur were 0.959±0.122 g/cm2 and 0.0±1.1, respectively.
2. Correlations between variables
Age was positively correlated with CACS and dietary calcium while it was negatively correlated with lumbar and femoral BMD (Table 2). CACS showed negative correlation with BMD (r=-0.280, P=0.049 with L1-4, r=-0.310, P=0.028 with femur neck, r=-0.333, P=0.018 with femur total) and hair calcium level (r=-0.352, P=0.012), while CACS did not showed any relationship with dietary calcium intake and serum creatinine level (Table 3). Serum calcium level was negatively correlated with lumbar BMD. Lumbar and femoral BMD were significantly correlated each other. Dietary calcium intake showed no significant relationship with other variables (Table 2, 3). Other serum variables were not correlated with CACS, BMD, and hair calcium level. Figure 2 showed summary of our study results.
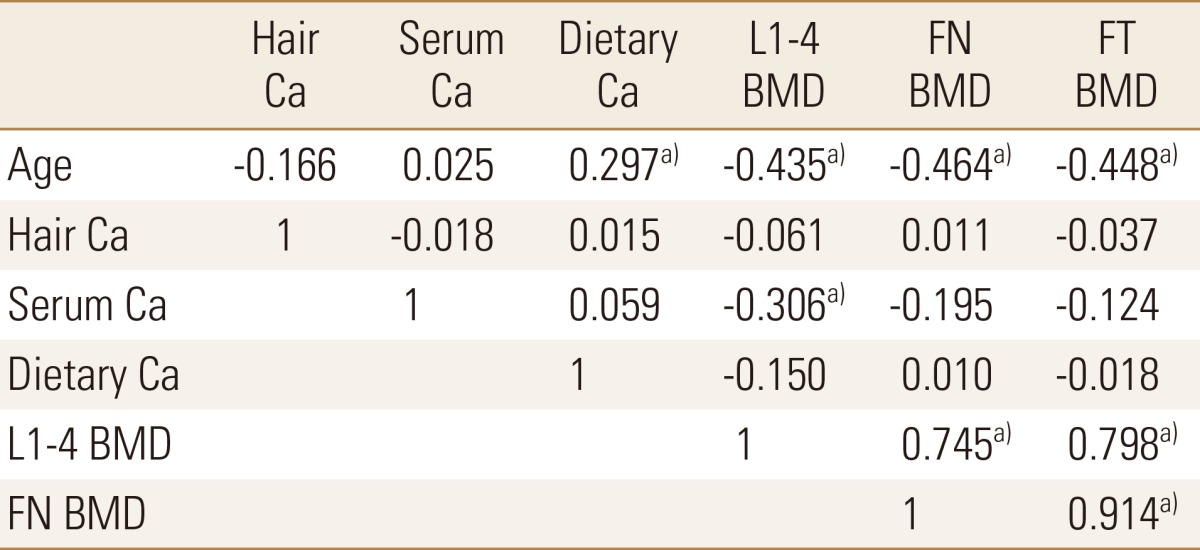
Pearson's correlation coefficients between age, hair and serum calcium level, dietary calcium intake and bone mineral density (n=50)

Spearman's correlation coefficients between coronary artery calcium score and other variables (n=50)
DISCUSSION
The aim of current study is to demonstrate interrelation among different calcium pools in body in order to see if a change in one calcium pool can affects the others. As a result, CACS was negatively related with BMD and hair calcium level in women.
Early study that investigated relationship between CACS and BMD reported negative result.[1920] However, many following researches reported inverse relationship between them.[212223] In these previous studies, subgroup analysis demonstrated that while BMD and CACS showed no significant relationship in men, but in women negative correlation was observed. It was consistent as our study. Some epidemiological studies suggested that estrogen deficiency is an independent risk factor for both osteoporosis and CVD.[2425] Bone and coronary artery is a common target organ of estrogen. Estrogen receptors have been demonstrated on coronary artery smooth muscle cell[26] as well as osteoblast[27] and osteoclast.[28] Another possible explanation connecting coronary artery disease and osteoporosis is atherosclerosis in blood vessel that distributes bone tissue. In contrast to atherosclerosis of large vessels that promotes calcification in vascular wall, atherosclerosis of the micro-vessels supplying bone tissue can promote age-related bone loss by increased osteoclast activity.[29] This process was partially mediated by oxidized lipids and other inflammatory factors.
In a previous epidemiologic study, hair calcium concentration and CVD mortality showed negative correlation.[17] CACS is well-known predictor of CVD risk, so the results were consistent with our study. The possible mechanism was competitive calcium deposition between vascular and intracellular calcium pools. In healthy body, vascular calcium pools are nearly empty, and intracellular calcium pools are maintained adequately by hormones such as parathyroid hormone (PTH) and vitamin D. But, in pro-atherosclerotic condition like systemic chronic low-grade inflammation induced by obesity, body calcium shifts into blood vessel walls, so calcium influx to intracellular pools can be decreased.
An observational study with premenopausal women showed no correlation between hair calcium level, BMD and calcium intake.[30] But another recent research reported hair calcium level is negative associated with BMD and calcium intake.[18] In our study, BMD, calcium intake and hair calcium level have no significant relationship. Menopause can be a significant factor of these different results. 56% of our study population was younger than 50 years old, so they are mostly in premenopausal status. So, our result is consistent with the previous observational study for premenopausal women. It is difficult to see the influence of main calcium reservoir - bone on hair calcium level in young women for calcium mobilization in premenopausal time is not as active as in postmenopausal period when calcium efflux due to bone resorption increases drastically.[31] In addition, we didn't check other hormones for calcium metabolism, such as PTH[32] and vitamin D.[33] These can be a confounding factor, too.
In our study, serum calcium concentration was negatively correlated with lumbar BMD. This is an incidental finding and there are no previous studies reported this relationship. Aging effect is possible explanation of this incidental finding. With aging, serum calcium input from bone increases, and regulation power of serum calcium concentration from hormones decreases. While, serum calcium concentration did not show significant relationship with CACS. By results from previous studies, circulating calcium levels in upper part of the normal range were associated with calcified plaque in the coronary arteries and CVD risk.[3435] But, these findings were usually significant at high risk group, such as male or high CACS. Although, our study population were relatively low risk group, with all female and low CACS.
Dietary calcium was not significantly correlated with lumbar BMD, but showed negative trend. This is inconsistent results compared with previous studies.[3637] Recall bias and small sample size can be the reason.
Our study has few limitations. First, it has retrospective design, which does not allow for the establishment of any causal relationships between the parameters. To evaluate precise relationship among different body calcium pools, longitudinal study should be conducted as a next step of our research. Also, we do not check menopausal status and calcium regulating hormones such as estrogen, PTH and vitamin D. To find more precise mechanism of calcium metabolism in body, further researches including menopausal status and calcium related hormones are required. Last, we cannot exclude the patients with osteophytes or osteochondrosis of lumbar spine. It can affect lumbar BMD when measured by DXA.
Nevertheless, our study presents several strong points: firstly, we originally elucidate relationship between CACS, BMD, hair/serum calcium level and calcium intake. Also, we use the precise measurement method like coronary MDCT and central DXA.
In conclusion, CACS has significant negative correlation BMD and hair calcium level in women. Different body calcium pools such as bone, hair and blood vessel significantly correlated each other. To research for relationship between cardiovascular risk and osteoporosis with aging, interactions of different body calcium pools should be taken into account.
Notes
No potential conflict of interest relevant to this article was reported.
The authors thank Song-Eun Young, a dietitian in Ajou University Hospital Health Promotion Center, for helping analysis of dietary nutrition, and Tae-Hee Kim, an assistant in Ajou University Hospital Medical Information System Team, for collecting laboratory data of the study.