Position Statement: Postmenopausal Osteoporosis Treatment Strategies in Korea
Article information
Abstract
Classifying patients with osteoporosis according to fracture risk and establishing adequate treatment strategies is crucial to effectively treat osteoporosis. The Korean Society for Bone and Mineral Research has issued a position statement regarding appropriate treatment strategies for postmenopausal osteoporosis. According to previous fragility fracture history, bone mineral density (BMD) test results, fracture risk assessment tool, and several clinical risk factors, fracture risk groups are classified into low, moderate, high, and very-high-risk groups. In high-risk groups, bisphosphonates (BPs) and denosumab are recommended as first-line therapies. Sequential BP treatment after denosumab discontinuation is required to prevent the rebound phenomenon. In the very high-risk group, anabolic drugs (teriparatide or romosozumab) are recommended as a first-line therapy; sequential therapy with antiresorptive agents is required to maintain BMD gain and reduce fracture risk. Fracture risk was reassessed annually, and the treatment plan was determined based on the results, according to the osteoporosis treatment algorithm for fracture risk.
INTRODUCTION
The prevalence of osteoporosis increases with age.[1] Osteoporotic fractures are associated with substantial morbidity and mortality, which can increase the burden of medical expenses.[2,3] Therefore, in an aging society, prevention and management of osteoporosis are important. Recently published guidelines classify patients with osteoporosis according to their risk of fracture (low-, moderate-, high-, and very high-risk) and recommend different treatment strategies according to the risk of fracture.[4–6] The algorithm for osteoporosis treatment was suggested, which included which drug to start according to the risk of fracture, duration of drug use, drug holiday period, and sequential treatment. Unlike previous guidelines, the very high fracture risk group was newly defined using specific criteria, including the recency of fractures, multiple fractures, very low bone mineral density (BMD), and several other clinical factors. In the very high-risk group, an immediate therapeutic response was required because the risk of subsequent fractures was high during the first year after the initial fracture. Thus, a more active and rapid osteoporosis treatment is recommended for very high-risk patients. The Korean Society for Bone and Mineral Research recommends appropriate postmenopausal osteoporosis treatment strategies for clinicians in Korea, considering the risk of fracture.
FRACTURE RISK STRATIFICATION
Fracture risk assessment should be performed before initiating osteoporosis treatment. The fracture risk categories are classified using previous fragility fracture history, BMD test results, fracture risk assessment tool (FRAX®), and some clinical risk factors. Fragility fractures are defined as fractures resulting from low-energy trauma, such as a fall from a standing height or lower, which would not ordinarily result in a fracture.[7] Fragility fractures can be classified into major (hip, spine, distal radius, and proximal humerus) and minor (pelvis, sacrum, ribs, distal femur, humerus, and ankle), according to their anatomic sites.[8] The fracture risk groups were low-, moderate-, high-, and very high-risk.
1. Low-risk group
Patients in the low-risk group should satisfy all of the following criteria:
- No previous fragility fractures, especially hip or spine fractures.
- BMD T-score ≥-1.0.
- FRAX-calculated 10-year hip fracture risk <3% and 10-year risk of major osteoporotic fractures <20%.
2. Moderate-risk group
Patients in the moderate-risk group should satisfy all of the following criteria:
- No previous fragility fractures, especially hip or spine fractures.
- −2.5 <BMD T-score <−1.0.
- FRAX-calculated 10-year hip fracture risk <3% and 10-year risk of major osteoporotic fractures <20%.
3. High-risk group
Patients are classified into the high-risk group if at least one of the following criteria is satisfied:
- History of fragility fractures, especially hip or spine fractures.
- BMD T-score ≤−2.5.
- FRAX-calculated 10-year hip fracture risk ≥3%, or 10-year risk of major osteoporotic fracture risk ≥20%.
4. Very-high-risk group
Patients are classified into the very high-risk group if at least one of the following criteria is satisfied:
- Recent fragility fractures, especially hip or spine fractures, within the past 12 months.
- Fractures occurring during approved osteoporosis treatment.
- Multiple fractures.
- Fracture occurred while on drugs that caused skeletal harm (e.g., glucocorticoids).
- BMD T-score <−3.0.
- High risk of falls or history of injurious falls.
- FRAX-calculated 10-year hip fracture risk ≥4.5% or 10-year risk of major osteoporotic fracture risk ≥30%.
OSTEOPOROSIS TREATMENT STRATEGIES ACCORDING TO FRACTURE RISK: APPROPRIATE INITIAL AND SEQUENTIAL THERAPY
After the initial fracture risk assessment, appropriate anti-osteoporotic agents are selected, and treatment is initiated depending on the risk of fracture. Fracture risk reassessment should be performed annually in all groups except the low-risk group. Depending on the results of fracture risk reassessment, appropriate sequential treatment is required. When treatment with anabolic agents is completed, subsequent treatment with antiresorptive agents should be considered to maintain improved BMD. If denosumab treatment is stopped, subsequent other antiresorptive agents should be administered to prevent rapid BMD loss and a rebound in bone turnover, and to decrease fracture risk. The algorithm used for the treatment of postmenopausal osteoporosis is summarized in Figure 1.
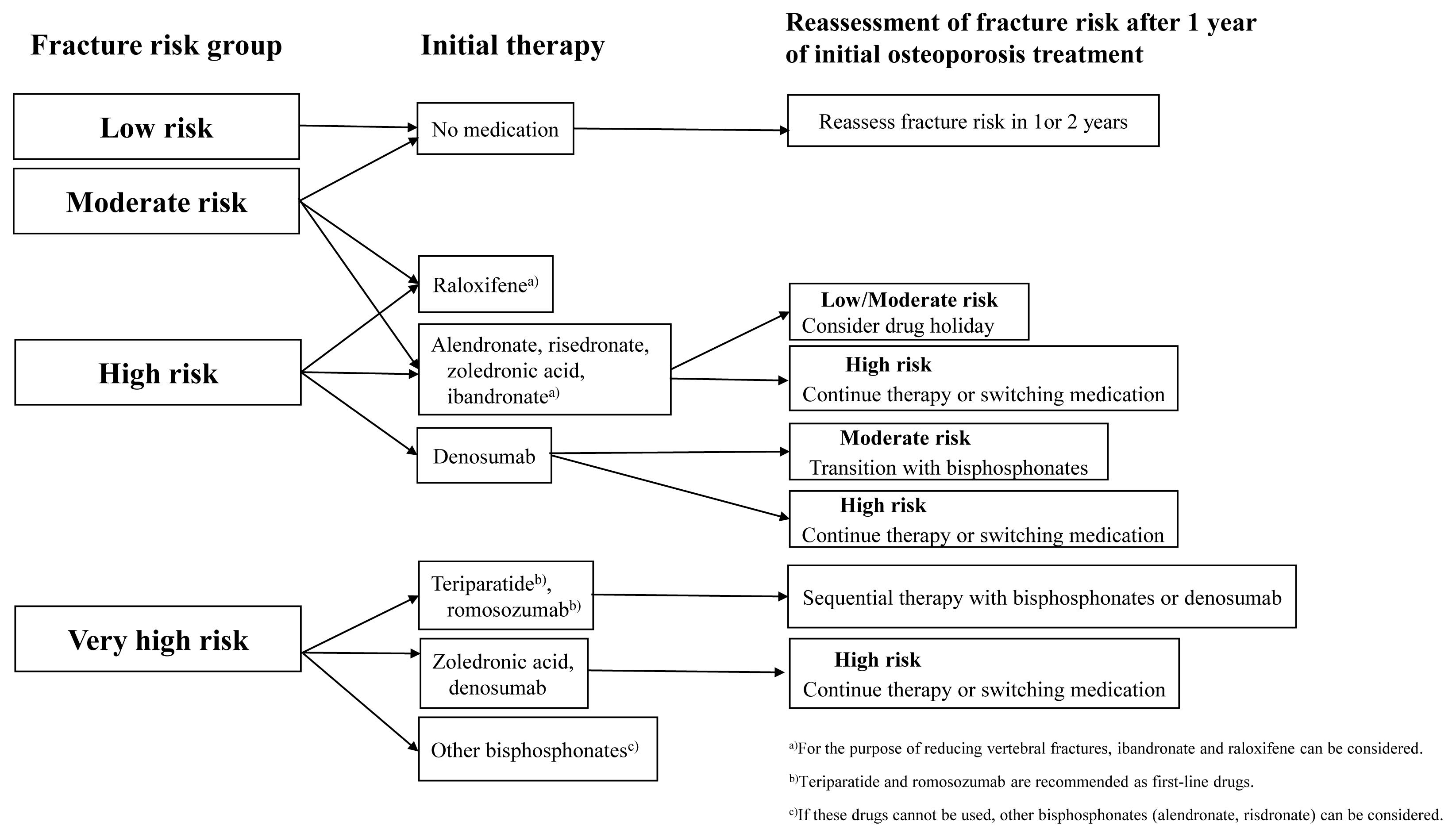
Algorithm for treatment of postmenopausal osteoporosis. “Low risk” includes no previous fragility fractures, especially hip or spine fractures, a bone mineral density (BMD) T-score above −1.0, and the fracture risk assessment tool (FRAX) calculated 10-year hip fracture risk <3% and 10-year risk of major osteoporotic fractures <20%. “Moderate risk” includes no previous fragility fractures, especially hip or spine fractures, −2.5 <BMD T-score <−1.0, and FRAX calculated 10-year hip fracture risk <3% and 10-year risk of major osteoporotic fractures <20%. “High risk” includes a fragility fractures, especially hip or spine fractures, or BMD T-score ≤−2.5 or FRAX calculated 10-year hip fracture risk ≥3%, or 10-year risk of major osteoporotic fracture risk ≥20%. “Very high risk” includes a recent fragility fractures, especially hip or spine fractures, within the past 12 months, or fractures occurring during approved osteoporosis treatment or multiple fractures, or fracture occurred while on drugs that caused skeletal harm, or BMD T-score <−3.0 or high risk of falls or history of injurious falls, or FRAX calculated 10-year hip fracture risk ≥4.5%, or 10-year risk of major osteoporotic fracture risk ≥30%.
1. Low-risk group
In the low-risk group, osteoporosis medication is not required and fracture risk reassessment is performed every 2 years.
2. Moderate-risk group
In the moderate-risk group, osteoporosis medication is not required and fracture risk reassessment is performed annually. Even in the moderate-risk group, if physicians determine that osteoporosis treatment is necessary, treatment with selective estrogen receptor modulators or bisphosphonates (BPs) (risedronate, etc.) might be considered.[9–14]
3. High-risk group
In the high-risk group, alendronate, risedronate, zoledronic acid, and denosumab are recommended as first-line therapies. As a result of meta-analysis including 107 randomized controlled trials (RCTs) that enrolled postmenopausal women with primary osteoporosis,[15] alendronate showed a 39% reduction in hip fracture risk (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.42–0.90), a 16% reduction in non-vertebral fracture risk (HR, 0.84; 95% CI, 0.74–0.94), and a 43% reduction in vertebral fracture risk (HR, 0.57; 95% CI, 0.45–0.71) compared to placebo. Risedronate demonstrated a 27% reduction in hip fracture risk (HR, 0.73; 95% CI, 0.58–0.92), a 22% reduction in non-vertebral fracture risk (HR, 0.78; 95% CI, 0.68–0.89), and a 39% reduction in vertebral fracture risk (HR, 0.61; 95% CI, 0.48–0.78) compared to placebo. Zoledronic acid showed a 40% reduction in hip fracture risk (HR, 0.6; 95% CI, 0.45–0.81), a 21% reduction in non-vertebral fracture risk (HR, 0.79; 95% CI, 0.67–0.94), and a 62% reduction in vertebral fracture risk (HR, 0.38; 95% CI, 0.25–0.58) compared to placebo.
While ibandronate and raloxifene reduced the vertebral fracture risk (HR, 0.67; 95% CI, 0.48–0.93; HR, 0.59; 95% CI, 0.46–0.76, respectively) compared to placebo, they did not reduce non-vertebral and hip fracture risk significantly.[15] Thus, ibandronate and raloxifene can be used to reduce the vertebral fracture risk, but they are not recommended to reduce the non-vertebral or hip fracture risk.
When BPs are used as a first-line therapy, BMD measurements and fracture risk reassessments should be performed annually. Oral BPs are typically used for 5 years, and intravenous BP are used for 3 years.[16,17] If the fracture risk is reduced to low-to-moderate moderate-risk after drug treatment, a drug holiday may be considered. During drug holidays, fracture risk reassessment should be performed at intervals of 1 to 2 years, and drug treatment should be resumed when the BMD T-score decreases significantly (T-score ≤−2.5) or a fracture occurs. Retreatment may be considered when bone resorption markers increase to pretreatment levels during drug holidays, but this is still controversial for general application. If the fracture risk persists in patients classified as high-risk, BPs should be continued or switching to a more effective drug should be considered. If oral BPs are used, switching to injectable antiresorptive agents can be considered. If injectable antiresorptive agents are used or the fracture risk increases to a very high level, switching to anabolic agents may be considered.
Denosumab showed a 44% reduction in hip fracture risk (HR, 0.56; 95% CI, 0.35–0.90), a 20% reduction in non-vertebral fracture risk (HR, 0.8; 95% CI, 0.67–0.96), and a 68% reduction in vertebral fracture risk (HR, 0.32; 95% CI, 0.22–0.45) compared to placebo in meta-analysis.[15] In postmenopausal women with osteoporosis, 10 years of denosumab treatment resulted in progressive increases in BMD and a lower incidence of fracture in comparison with the placebo group in the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) extension study.[18] However, the discontinuation of denosumab treatment is associated with rapid bone loss.[19] After denosumab discontinuation, BMD at all skeletal sites declines significantly and returns to pretreatment values after 1 to 2 years denosumab discontinuation.[20,21] Suppressed bone turnover markers, C-terminal telopeptide and propeptide of type I collagen, increase above pretreatment levels within 3 and 6 months after denosumab discontinuation.[19] And it has been reported that the risk of multiple vertebral fractures is increased in patients who discontinue denosumab.[22,23] In real-world settings, the risk of any fracture, vertebral fracture and multiple vertebral fractures increased in patients discontinuing denosumab.[24,25] Sequent BPs treatment (alendronate, zoledronic acid) after denosumab discontinuation effectively maintained the BMD gain obtained with denosumab treatment.[26–29] Thus, if denosumab is used as first-line therapy, treatment is continued until the fracture risk is reduced to moderate; when discontinuation of denosumab is considered, subsequent BPs treatment is required. The evidence for sequential raloxifene treatment after denosumab discontinuation to prevent rebound phenomenon is insufficient.[30]
4. Very-high-risk
In very-high-risk patients, anabolic agents, such as teriparatide, abaloparatide or romosozumab, are strongly recommended as first-line therapies. However, the abaloparatide has yet to be approved for use in South Korea. In a meta-analysis, teriparatide showed a 38% reduction in non-vertebral fracture risk (HR, 0.62; 95% CI, 0.47–0.80) and a 73% reduction in vertebral fracture risk (HR, 0.27; 95% CI, 0.19–38) compared to placebo.[15] The effect of teriparatide on reducing hip fractures was not significant, but another meta-analysis of 23 RCTs showed that teriparatide reduced hip fractures by 56% compared to control.[31] In this meta-analysis, control group included patients with placebo or other medications, and not only postmenopausal women but also men and patients with glucocorticoid-induced osteoporosis were involved, so there may be differences from previous study results. In the VERO trial, teriparatide reduced the risk of new vertebral and clinical fractures among postmenopausal women with severe osteoporosis compared to risedronate.[32] BMD declines quickly after discontinuation of teriparatide,[33] and sequent BPs or denosumab therapy prevent bone loss and further increase BMD.[33,34]
The FRAME trial, romosozumab treatment for 12 months was associated with a lower risk of new vertebral fractures and higher spine and hip BMD than placebo treatment.[35] One year of romosozumab treatment followed by denosumab maintains the fracture reduction benefit and increases the spine and hip BMD.[35,36] In the ARCH trial, romosozumab treatment for 12 months, followed by alendronate, resulted in a significantly lower risk of fracture than alendronate treatment alone in postmenopausal women with osteoporosis.[37] Thus, after one year of romosozumab treatment, sequential treatment with antiresorptive agents is required to maintain increased BMD and reduce fracture risk.
Denosumab or zoledronic acid, which are strong antiresorptive agents, can also be used as a first-line therapy in very high-risk groups. If denosumab or zoledronic acid is difficult to administer, other BPs (alendronate and risedronate) can be considered. Administration of denosumab or zoledronic acid in the very high-risk group is the same as treatment in the high-risk group. Treatment with zoledronic acid can be continued for 6 years, and if bone loss progresses or fractures occur repeatedly, treatment may potentially be changed to denosumab, teriparatide or romosozumab.
Teriparatide treatment in patients previously treated with BP or denosumab increased spinal BMD but decreased hip BMD.[34,38] In the STRUCTURE trial, romosozumab increased hip and spine BMD and estimated hip strength compared to teriparatide in women with postmenopausal osteoporosis transitioning from BP therapy.[37] Transitioning to romosozumab after 12 months of denosumab treatment improves spine BMD and maintains total hip BMD; however, the decreased levels of bone turnover markers during denosumab administration gradually return to baseline before denosumab administration.[39] Switching from an anabolic agent to an antiresorptive agent is recommended in the very-high-risk group rather than switching from an antiresorptive agent to an anabolic agent, as it shows a much more effective increase in spine and hip BMD and a reduction in fracture risk.
It is important to treat osteoporosis appropriately according to the fracture risk, but, in Korea, fracture risk assessment and osteoporosis treatment, especially anabolic agents are limited in insurance coverage. This is a part that needs to be improved so that many patients can benefit.
CONCLUSION
It is important to classify postmenopausal patients with osteoporosis according to the risk of fracture and establish a treatment strategy. In high-risk patients, BPs or denosumab are recommended as first-line therapy. Moreover, in very high-risk patients, anabolic drugs (teriparatide or romosozumab) are recommended as the first-line therapy and sequential therapy with antiresorptive agents is needed.
Notes
Ethics approval and consent to participate
Not applicable.
Conflict of interest
No potential conflict of interest relevant to this article was reported.
