The Association of Coronary Artery Calcium Score and Osteoporosis in Postmenopausal Women: A Cross-Sectional Study
Article information
Abstract
Background
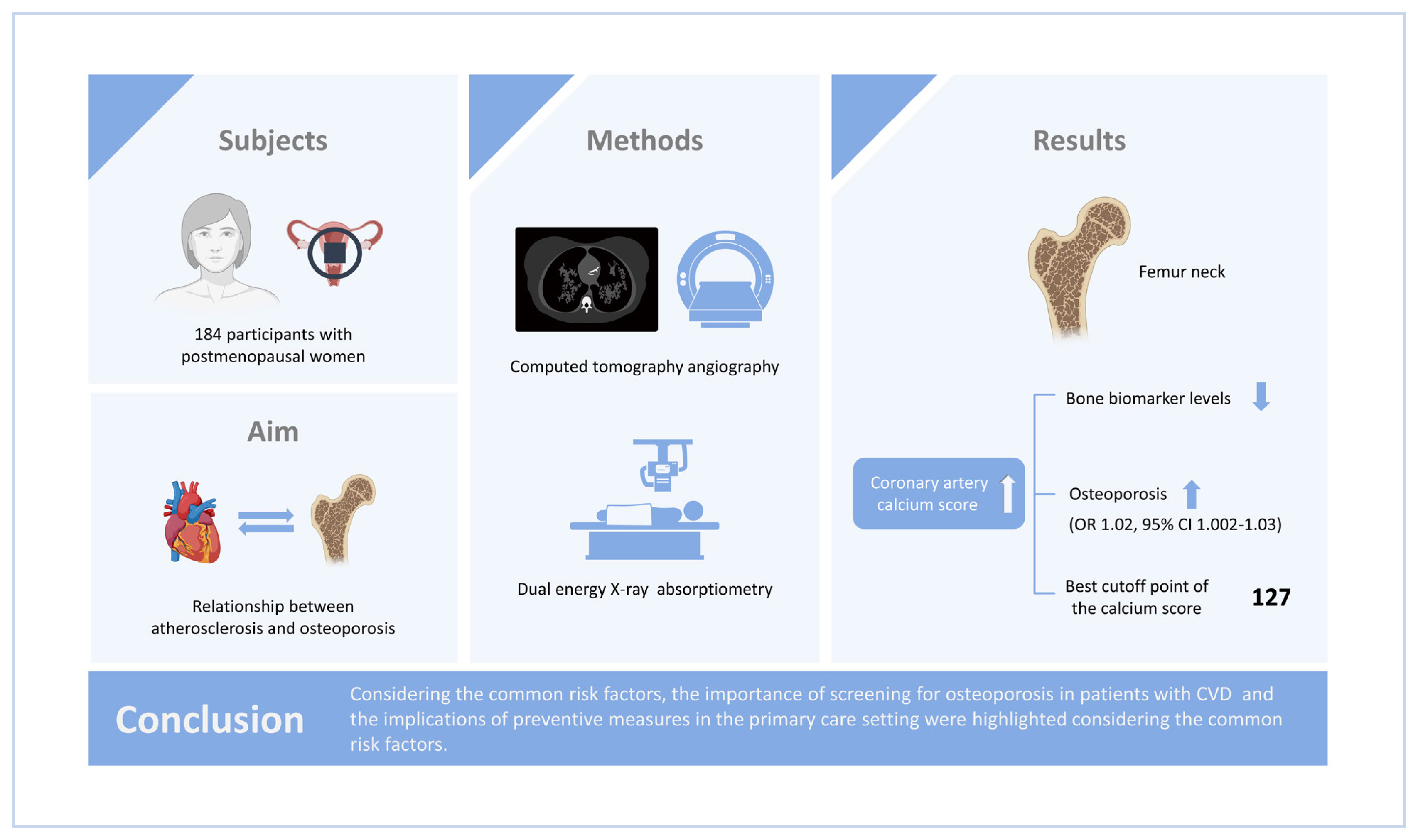
The association between osteoporosis, a common metabolic bone disorder, and atherosclerosis has been reported in different studies. In this study, we aimed to investigate the association between the coronary artery calcium score (CACS) and bone mineral density (BMD) at different sites and bone biomarkers in postmenopausal women.
Methods
A total of 184 participants were enrolled in this study. The CACS and BMD at different sites, including the spinal, total hip, and femoral neck, were measured using computed tomography angiography and dual energy X-ray absorptiometry, respectively. Serum levels of osteocalcin, β-C-terminal telopeptide (β-CTX), parathyroid hormone, and 25-hydroxy-vitamin D were measured.
Results
A negative association between CACS and bone biomarker levels (osteocalcin, P=0.021; β-CTX, P=0.013) was noted. The univariable model showed an association between CACS and osteoporosis of the femoral neck (P=0.03). It was found that with an increase of 10 U in CACS, the odds of osteoporosis at the femoral neck escalates by 2% (odds ratio=1.02, 95% confidence interval, 1.002–1.03) using the multivariate logistic regression model, while such an association with osteoporosis could not be found at the spinal site. The best cutoff point of the calcium score was estimated to be 127.
Conclusions
The results suggest that in postmenopausal women, coronary atherosclerosis is independently associated with osteoporosis of the femoral neck, but such an association could not be detected with spinal osteoporosis. The importance of screening for osteoporosis in patients with cardiovascular disease and the implications of preventive measures in the primary care setting were highlighted considering the common risk factors.
INTRODUCTION
Osteoporosis and coronary artery disease (CAD) are common chronic conditions in the elderly population and result in high morbidity and mortality.[1] It is estimated that annually 9 million osteoporotic fractures occur throughout the world.[1] Keeping this in mind and the increasing number of the elderly population in the world is enough to get aware of the high burden of these 2 conditions on the health systems.
There seem to be common pathophysiological characteristics as well as shared risk factors in both diseases, including hypertension, menopause, smoking, alcohol consumption, and lifestyle.[2] There is growing clinical evidence on the association between low bone mass and cardiovascular diseases (CVDs), and endothelial dysfunction.[3,4] The association between low bone mineral density (BMD) and CAD,[5,6] especially in postmenopausal women and elderly men over 50,[7] cortical bone loss and progression of aortic calcification,[8] and the risk of nonvertebral fractures and carotid plaques in elderly women [9] have been reported in several epidemiological studies. The common pathophysiologic feature in both diseases is calcification that is under the control of inflammation and oxidative stress. High serum levels of inflammatory mediators including C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), osteoprotegerin, and bone morphogenetic proteins showed association with atherosclerosis and osteoporosis; however, the main common causative agent of the pathway is not fully understood.[10] Many proteins have been produced in both sites (coronary artery and bone) including osteocalcin and osteopontin [10,11] by which the calcification is initiated and controlled. Osteocalcin is a noncollagenous protein and a bone formation marker, and also a marker of vascular calcification.[12] Osteocalcin is a pleiotropic protein having a regulating role in the bone as well as endothelial homeostasis; however, its association with cardiovascular events and death is unclear and U-shaped.[13] β-C-terminal telopeptide (β-CTX) is a bone resorption biomarker and is a byproduct of β-collagen degradation.[14] Both osteocalcin and β-CTX could be indicative of higher cardiovascular risk and mortality.[15] Likewise, osteopontin and osteocalcin as 2 bone markers and bone metabolism regulators have been found in calcified plaques in coronary arteries.[16]
Osteoporosis is a silent disease that is mostly diagnosed after developing fracture [17]; so, early diagnosis and treatment are essential in reducing its morbidity and mortality. The primary prevention often is directed toward lifestyle modification, receiving proper sources of calcium and vitamin D, and weight-bearing aerobic exercise; while searching into the common features and pathophysiological factors between osteoporosis and the other senile chronic diseases like CVDs may shed light on our pathway to primary prevention.
Coronary artery calcium score (CACS) is a strong predictor of the overall atherosclerotic burden and correlates with cardiovascular mortality.[18] Recently CACS measurement is recommended for screening asymptomatic individuals with intermediate-risk.[19] Bone health is evaluated by measuring BMD at different sites by dual energy X-ray absorptiometry (DXA), the gold standard test. Despite the similarities in both diseases’ pathophysiology, there is no conclusive evidence suggesting the potential association.
Previously, in a pilot study on 11 postmenopausal women who had CACS higher than 80, we observed the potential association between CACS and osteocalcin, femoral bone density, and T-score.[10] Also, several large studies have presented the association between CACS and osteoporosis [9,20–22]; however, the results are not conclusive. This study is different from our pilot study because we did not consider a limit for CACS. In this study, we aimed to determine the association between CACS with osteoporosis at different sites and bone biomarkers in postmenopausal women.
METHODS
1. Participants
The study was performed from April 2017 until December 2019. Two hundred postmenopausal women who have taken coronary computed tomography (CT) scanning with (Dual Source Flash-128 slice) for any reason were enrolled in the study. After CT angiography, the study participants were informed about the study and after giving verbal informed consent they were introduced to the outpatient clinic of Diabetes Research Center of the Endocrinology and Metabolism Research Institute of Tehran University of Medical Sciences for enrollment. After giving written informed consent, the patients’ anthropometrical measurements, health profile, and drug history were recorded including age, weight, height, years since menopause, smoking, addiction, alcohol drinking, history of fracture in herself or her parents, and past medical history. The patients were asked to report their smoking status and alcohol consumption. The participants with a history of cancer, acute infection, endocrine disorders, use of special medications such as hormones, corticosteroids, gonadotropin releasing hormone analogs, anticonvulsant drugs, heparin, aluminum-containing antacids, anti-osteoporotic drugs, and thyroid hormones were excluded. Bone densitometry and blood sampling were done for all enrolled participants. The study was conducted according to the Declaration of Helsinki and after approval of the research ethics committee of the Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.EMRI.REC.1396.00171). All participants signed the informed consent after receiving information about the study.
2. Clinical assessment
The patients’ CACS was calculated using Agatston et al. [23] method by an experienced radiologist. Each participant’s bone densitometry was performed by DXA (Hologic Inc., Bedford, MA, USA) at different sites including the lumbar spine, total hip, and femoral neck. The BMD measurement was done using a QDR 4500 A fan-beam densitometer. The study participants changed into disposable gowns and removed all pieces of jewelry and everything that may interfere with the DXA measurement. Using the industry-standard techniques and Hologic APEX software version 3.3, the results were reviewed and analyzed.
Blood samples were collected at fasting state from the antecubital vein and centrifuged at 10,000 g for 10 min and kept at -70˚C until analysis. The serum concentration of 2 bone markers, including osteocalcin, and β-CTX were measured by the Electrochemiluminescence method using Cobas e411 (Roche Diagnostics, Mannheim, Germany). Serum 25-hydroxy-vitamin D (25[OH]D) was measured by the enzyme-linked immunosorbent assay method (Infinitum Biotech, El Cajon, CA, USA). Intra- and inter-assay precision expressed as coefficient of variations (CV%) were between 1.1% to 1.9% and 4.1% to 5.4% for osteocalcin, 1.2% to 4.9% and 3.3% to 6.3% for β-CTX and 2.1 to 4.2 and 3.4% to 7.1% for 25(OH)D, obtained by examination of quality control materials (in different concentrations) provided by the manufacturers.
Osteoporosis is defined by the World Health Organization (WHO) [24] as having a T-score less than -2.5 at any bone site including femur neck, spine, or total hip; while T-score between −1 and −2.5 is considered osteopenia. Fracture risk assessment tool (FRAX), which was developed by the WHO and provided by the University of Sheffield [25], was calculated for all participants using a computer-driven FRAX tool for Iranians. This tool uses clinical risk factors and BMD at the femoral neck for computation and estimates the risk of fracture in the next 10 years.[26]
3. Statistical analysis
We used the number (percent) for the categorical and the mean (± standard deviation) for the normally distributed continuous variables to describe the summary statistics. The normality was assessed using the Shapiro–Wilk test. The variables with non-normal distribution were presented as the median and interquartile range. Considering the osteoporosis status, the participants were compared for the baseline characteristics using the χ2 test for the categorical variables, independent sample t-test for normally distributed variables, and Mann-Whitney U test for the non-normal ones. Spearman rank test was applied to assess the correlation between calcium score and BMD, trabecular bone score (TBS), and bone turnover markers. Using the logistic regression model, we investigated the associates of osteoporosis. All potential variables with P-value lower than 0.2 in the univariable model were selected for adjusting in the multivariable model. Considering the limited sample size, to adjust for the most important variables, we used the FRAX risk score (without BMD) that contains all traditional risk factors of osteoporosis including age, body mass index, current Smoking, Glucocorticoids consumption, alcohol consumption and history of rheumatoid arthritis, secondary osteoporosis. We used femoral neck osteoporosis and spinal osteoporosis as the response variable regarding the limited number of osteoporosis at the total hip. We used the Akaike Information Criterion to show the discrimination power of the model. Using the Liu [27] method, the best cut point of calcium score was estimated, maximizing the product of the sensitivity and specificity. We performed statistical analyses using the STATA 14.0 software (Stata Corp., College Station, TX, USA).
RESULTS
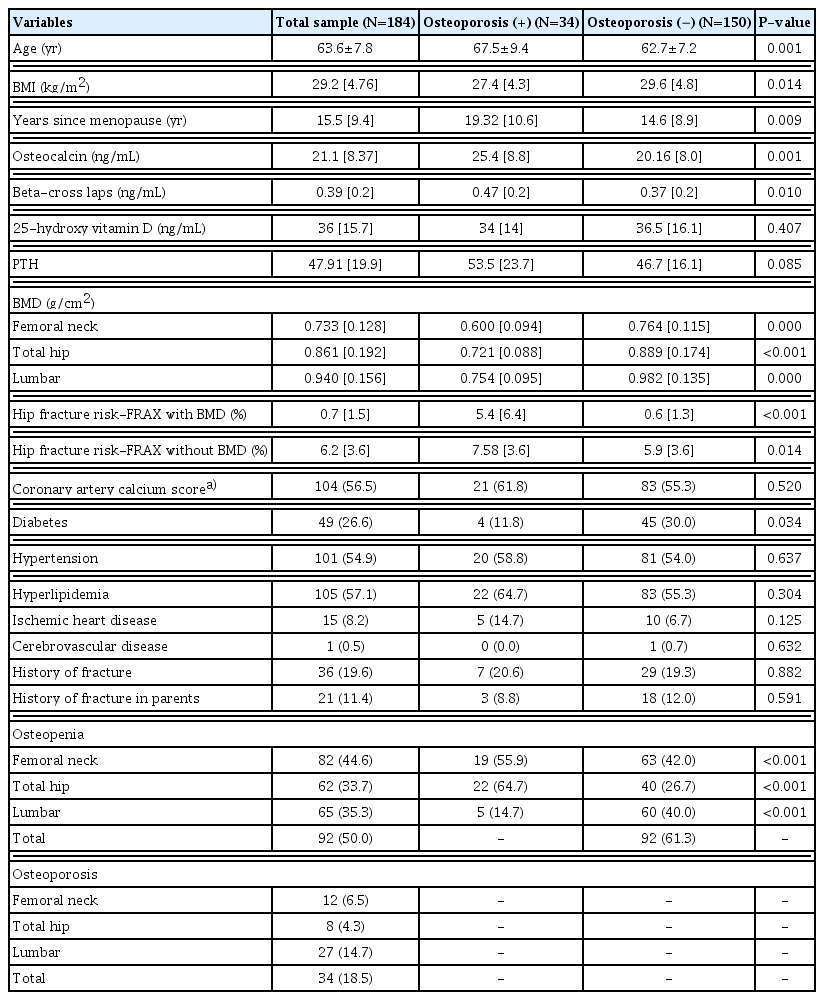
A total of 184 out of 200 postmenopausal women with a mean age of 63.6±7.8 years were included in the study; because 16 women canceled their appointment for densitometry after enrollment. In all, 18.5% (N=34) of participants had osteoporosis in at least one site. Osteopenia was detected in 50.0% (N=92) of study population. Table 1 shows the characteristics of study participants. As it shows, significant differences were detected in the level of bone turnover markers.
Considering the CACS score above zero as pathologic, 56.5% of participants were categorized as pathologic. There was a negative correlation between CACS and osteocalcin (Spearman’s rho, −0.170; P=0.021), and β-CTX (Spearman’s rho, −0.183; P=0.013). No significant correlation was detected between CACS score and BMD in lumbar (Spearman’s rho, 0.038; P=0.606), femoral neck (Spearman’s rho, −0.124; P=0.100) or total hip (Spearman’s rho, −0.0957; P=0.199), while in women who had information about TBS (N=67), a negative and significant correlation was showed for TBS (Spearman’s rho, −0.253; P=0.039). A positive significant correlation was also found between CACS score and the hip fracture risks; ([FRAX without BMD, Spearman’s rho, 0.351; P≤0.001], [FRAX with BMD, Spearman’s rho, 0.246; P=0.001]). The univariable model shows the significant association between CACS score and osteoporosis at the femoral neck (odds ratio [OR], 1.02; P=0.031) while the association was not detected with the spinal site (OR, 1.00; P= 0.990).
Table 2 shows the results of logistic regression models, considering femoral neck osteoporosis as the response. According to the results of the univariable model, the FRAX risk score, CACS score and osteocalcin were selected to include in the multivariable model. The results showed that increasing 10 scores to the CACS increases the odds of osteoporosis by 2%. The model resulted an area under the curve (AUC) of 0.791 (0.635–0.948) that means a discriminatory power of almost 80% of individuals with osteoporosis (Fig. 1). By maximizing the sensitivity (0.75) and specificity (0.77), the best cut-point of the calcium score was estimated as 127.
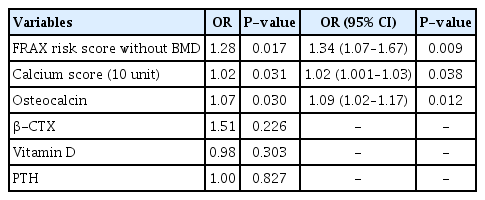
The results of univariable and multivariable logistic regression model considering the femoral neck osteoporosis as the response variable
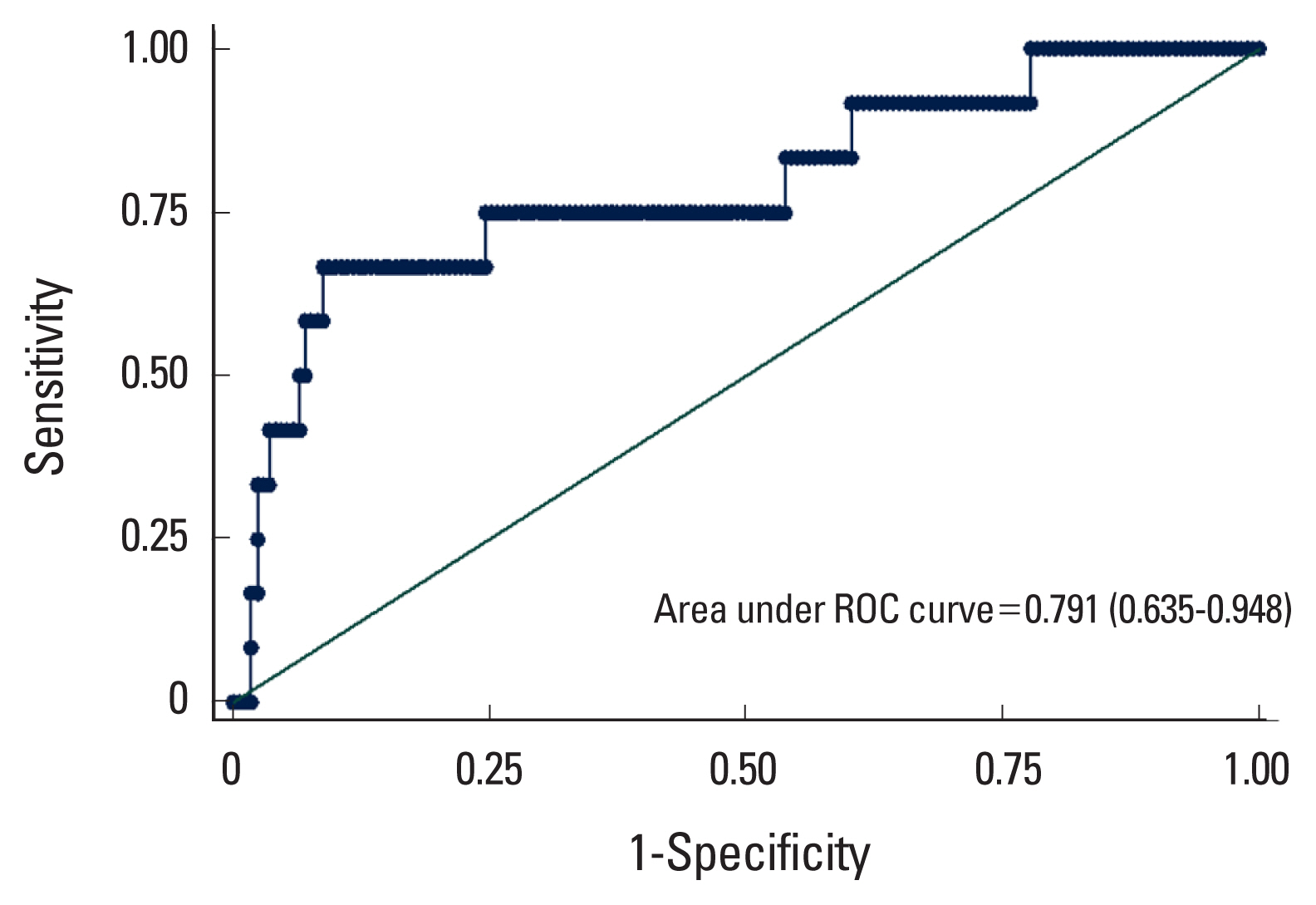
The area under the receiver operating characteristic (ROC) curves of the multivariable model (fracture risk assessment tool [FRAX] + osteocalcin + calcium score).
The optimal cutoff point and its diagnostic performance including sensitivity, specificity, positive predictive value, and negative predictive value for osteoporosis at femoral neck and spine was presented in Table 3.
DISCUSSION
This study investigated the association between CACS, bone biomarkers including osteocalcin and β-CTX, and osteoporosis in postmenopausal women. Our results showed the association between osteoporosis at the femoral neck and CACS in study participants. Also, we observed that ten units increase in calcium score escalates the odds of osteoporosis by 2%; this finding has the discriminatory power of 80%.
Atherosclerosis and osteoporosis are 2 major prevalent chronic diseases in postmenopausal women with high morbidity and mortality [28,29]; while, their exact association has not been fully understood. Our results show the association between CACS and osteoporosis at the femoral neck while we could find no such association at the spine. In line with our findings, Xu et al. [30] reported the association of low BMD at the femoral neck with an increased risk of developing coronary lesions in postmenopausal women.
Choi et al. [31] observed an increased CACS and plaque burden in women with low BMD at lumbar spine and femur regardless of age and vascular risk factors; however, they considered 0 as the CACS cut-off point. Accordingly, the Framingham Offspring Study indicated a significant association between L3 vertebral bone density (VBD) and CACS in women of 60 years.[9] This association in women was confirmed by the Multi-Ethnic Study of Atherosclerosis.[32] In a retrospective study, Lee et al. [33] found an association between low BMD and high CACS, which stayed significant after adjusting for age, and CVD risk factors. Besides, they reported an association between osteoporosis and obstructive CAD as well as a 9-fold increase in the risk of multivessel disease in osteoporotic postmenopausal women which is in agreement with the Ahmadi et al. [21] study that showed the same results regardless of age, gender, ethnicity, and risk factors. However, Chan et al. [9] reported the inverse association between CACS and trabecular volumetric BMD in women.
Although most studies have been conducted in women, especially in postmenopausal women, an international multi-ethnic study reported a significant inverse association between vertebral bone density (VBD) and coronary atherosclerosis in middle-aged men.[34]
In contrast, the Rotterdam Study found no association between BMD and CACS in men and women (mean age, 64 years).[35] Likewise, the Study of Womens’ Health Across the Nation did not show a significant association between BMD and CACS in premenopausal women.[36]
The mechanism of the association between CACS and osteoporosis is unknown and the role of common biomarkers is under debate. The present study showed a higher serum level of osteocalcin and β-CTX in osteoporotic women and an inverse correlation between CACS and these bone biomarkers. Although a potential inverse correlation between osteocalcin, β-CTX, and CACS has been presented in this study, the findings are controversial, and the exact role of bone markers in atherosclerosis needs to be further defined. Our findings, are inconsistent with our previous pilot study in which we found a positive association between CACS, osteocalcin, and β-CTX.[10] β-CTX has been shown to be present in areas of intimal hyperplasia and in advanced plaques.[37] It has been shown that rising concentrations of β-CTX is associated with increased risk of hip fractures and a significant inverse relationship with hip BMD.[38] Also, there are evidences of the independent association of high β-CTX with increased risk of cardiovascular mortality.[15] Furthermore, a lower level of osteocalcin was observed in patients with atherosclerosis compared to those without atherosclerosis.[39,40] In a recent study in Chinese diabetic patients, Lv et al. [41] reported an inverse correlation between serum osteocalcin and lower extremity atherosclerotic disease. It is supposed that the uncarboxylated osteocalcin as the active form of osteocalcin may be a more useful biomarker, but this hypothesis has yet to be studied. The studies show that the serum level of uncarboxylated osteocalcin is inversely associated with the abdominal aortic calcification score in men.[42] Likewise, a lower level of uncarboxylated osteocalcin was significantly associated with CAD.[43] In a meta-analysis, Millar et al. [44] determined that more positive relationship between osteocalcin and measurements of atherosclerosis or calcification have been observed in male than female. Furthermore, the negative relationship between osteocalcin and calcification have been found in Asia rather than Europe and America [44]; however, overally they showed no certain association in between.[44]
In the current study, adding the serum level of vitamin D did not show significant effect on the association between CACS and osteoporosis; albeit vitamin D increased the AUC of our proposed model. The studies on the effect of vitamin D supplementation and the risk of fractures are controversial; some reported it as protective, some others showed no effect and one study reported that supplementation with vitamin D increases the risk of fractures.[45]
Several mechanisms explain the relationship between atherosclerosis and osteoporosis. This relationship is mediated by inflammatory mediators which are the byproduct of lipid oxidation and disposition in vascular subendothelium and osteolysis.[46,47] Nevertheless, the inflammatory mediators and cytokines such as TNF-α, CRP, and IL-6, may have a paradoxical effect on different tissues that complicates our understanding of the exact pathophysiological pathway. Furthermore, in postmenopausal women, estrogen deficiency was proposed by Manson et al. [48] as a joint mediator in CAD and osteoporosis. Estrogen has common receptors on bone cells, including osteoblasts and osteoclasts as well as on smooth muscle cells and blood vessels.[36] Likewise, the results of our study and Lee et al.’s study [33] support this theory.
Our study indicated the best cut-off point of calcium score as 127, while Lee et al. [33] considered the cut-off point as 100. However, Rumberger et al. [49] proposed that a calcium score equal to or above 80 may demonstrate 84% sensitivity and specificity for more than 50% of stenosis. Interestingly, our model shows that a ten units increase in CACS increases the chance of osteoporosis by 2%.
The novelty of this study is finding the negative correlation of bone biomarkers with CACS simultaneously. The higher level of both osteocalcin and β-CTX in osteoporotic patients confirms the higher rate of bone metabolism in our osteoporotic patients which were in inverse correlation with CACS. This finding in addition to the association of CACS with osteoporosis potentiates our hypothesis of a common pathophysiological mechanism of osteoporosis and atherosclerosis. This hypothesis should be further evaluated in a larger sample size.
In discussing the results, we should consider the measurement methods by which we measured CACS and BMD; CT angiography, and DXA, respectively. Because the measurement methods may be responsible for different results of different studies. Furthermore, the gender of the participants could be of significant importance in discordant results.
Concerning the strengths of the present study, the use of CT imaging in detecting vascular calcification and Agatston Score are sensitive methods to show coronary artery calcification.[50,51] Furthermore, the medication history of all participants was recorded, and the exclusion criteria were strictly taken into account in subject enrollment.
The sample size of the study could be considered as the major limitation. In addition, the current study was cross-sectional, which cannot show causality. As the study was conducted on postmenopausal Iranian women, our results may not be generalizable in another gender, age and ethnic groups. The effect of probable uncontrolled confoundings such as physical activity and food intake and causation should not be ignored. Despite these limitations, this study emphasizes the association between osteoporosis and CAD in postmenopausal women.
CONCLUSIONS
In conclusion, high CACS and its association with osteoporosis in postmenopausal women could be of clinical importance. The findings of this study show that in postmenopausal women coronary atherosclerosis is independently associated with osteoporosis at the femoral neck. Also, each 10 unit increase in CACS escalates the risk of osteoporosis by 2% which has an acceptable discriminatory power. The findings of this study may help in having a better understanding of the importance of screening osteoporosis followed by the implication of preventive measures in high-risk populations to prevent the related fractures. Further studies by large sample size and in different gender are needed to show the casualty.
Notes
Funding
Research reported in this publication was supported by Elite Researcher Grant Committee under award number (958346) from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran. In addition, the study was performed with the financial support of the and Endocrinology and Metabolism Research Institute, Osteoporosis Research Center of Tehran University of Medical Sciences, Tehran, Iran.
Authors’ contributions
Conceptualization: MA and PS; Data curation: FR, NF, SS, and GB; Formal analysis: MA, SS; Methodology: FR and NF; Writing - original draft: MA, FR, NF, and PS; Writing - review & editing: MA, FR, NF, SS, GB, and PS; All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Research Ethics Committee of the Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.EMRI.REC.1396.00171).
Conflict of interest
No potential conflict of interest relevant to this article was reported.