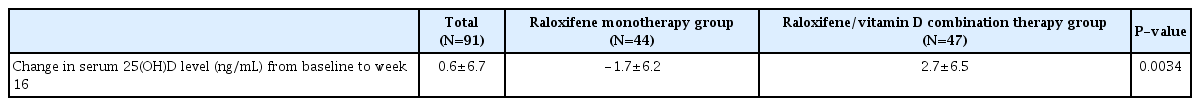Raloxifene/Vitamin D Combination Therapy vs. Raloxifene Monotherapy on Serum 25-Hydroxy-Vitamin D Level among Postmenopausal Women with Osteoporosis or Osteopenia: A Randomized Controlled Trial
Article information
Abstract
Background
We compared the efficacy of a fixed dose combination of raloxifene 60 mg/vitamin D 800 IU to raloxifene 60 mg alone on vitamin D status, as indicated by change in serum 25-hydroxy-vitamin D (25[OH]D) levels.
Methods
In this 16-week, open-label, randomized, active controlled, multicenter clinical trial conducted in 4 university-affiliated hospitals in Korea, postmenopausal women aged 55 to 70 years with osteoporosis or osteopenia were randomly assigned in a 1:1 ratio to receive raloxifene 60 mg/cholecalciferol 800 IU combination therapy or raloxifene 60 mg monotherapy. Primary endpoint was change in serum 25(OH)D level from baseline to 16 weeks after the intervention.
Results
A total of 96 participants were randomly assigned to raloxifene/vitamin D combination therapy (N=49) and raloxifene monotherapy (N=47) groups. At week 16, serum 25(OH)D level increased from baseline, only in the raloxifene/vitamin D combination therapy group. Change in serum 25(OH)D level from baseline to week 16 was higher in the raloxifene/vitamin D combination therapy group (2.7±6.5 ng/mL) than in the raloxifene monotherapy (−1.7±6.2 ng/mL; P=0.0034) group. Proportions and number of adverse events (AEs) categorized by the System-Organ Class were not different between the groups. There was only one severe AE case (spondylolisthesis; raloxifene/vitamin D group), unlikely to be related to trial intervention.
Conclusions
Among postmenopausal women with osteoporosis or osteopenia, a fixed dose combination of raloxifene 60 mg/vitamin D 800 IU showed superior efficacy in elevating serum 25(OH)D levels compared with raloxifene 60 mg alone during 16 weeks of follow-up. The safety of raloxifene/vitamin D combination was comparable to raloxifene alone.
INTRODUCTION
Raloxifene is a selective estrogen receptor modulator (SERM), which has antiresorptive activity.[1,2] Agonistic actions on bone and lipid metabolism and antagonistic effects on endometrium and breast in estrogenic pathways have been reported with this medication.[3,4] Raloxifene is used for the treatment of osteoporosis or osteopenia in postmenopausal women as it reduces bone loss and risk of vertebral fracture in this population.[3,5–7]
Adequate vitamin D status is an important component of bone health.[8–10] Vitamin D promotes absorption of calcium and phosphate from intestine, which can be used for bone mineralization.[10] A direct effect of vitamin D on bone cells has also been reported,[10] and optimal vitamin D repletion seems to maximize the response to anti-resorptive agents including raloxifene in postmenopausal women.[11] Therefore, oral vitamin D intake of 800 to 1,000 IU a day is recommended, and a serum 25-hydroxy-vitamin D (25[OH]D) level greater than 20 ng/mL is generally suggested to prevent osteoporosis.[10,12] In addition, a serum 25(OH)D level greater than 30 ng/mL is considered probably helpful to manage osteoporosis and prevent fractures and falls.[10,12] Nevertheless, South Korea has shown the highest prevalence of vitamin D inadequacy in a multinational study including 2,606 osteoporotic postmenopausal women from 18 countries.[13] Furthermore, in a large international study of postmenopausal women with osteoporosis in Eastern Asia that found a high prevalence of vitamin D inadequacy, defined as serum 25(OH)D level <30 ng/mL, the prevalence was 92% in South Korea.[14]
Considering the key role of vitamin D status for bone health and high prevalence of vitamin D inadequacy among postmenopausal women with low bone density, daily supplementation of cholecalciferol can be a practical and efficient strategy to improve vitamin D status in this population. Furthermore, combination therapy with a single capsule containing active drug and vitamin D supplements can be a useful tool for women with osteoporosis or osteopenia in terms of convenience and adherence of patients. A fixed dose combination of raloxifene 60 mg and vitamin D 800 IU (Rabone D Cap.; Hanmi Pharm, Seoul, Korea) has been introduced to provide both vitamin D and raloxifene in a single capsule to postmenopausal women with osteoporosis or osteopenia. Through this 16-week, open-label, randomized, active controlled trial, we directly compared a fixed dose combination of raloxifene 60 mg and vitamin D 800 IU to raloxifene 60 mg alone without vitamin D supplementation. The primary aim was to assess the effects of treatment on vitamin D status as indicated by change in serum 25(OH)D levels.
METHODS
1. Study design
This is a 16-week, open-label, randomized, active controlled, multicenter clinical trial conducted in 4 university-affiliated hospitals (Samsung Medical Center, Yeouido St. Mary’s Hospital, Kyung Hee University Hospital at Gangdong, and Soonchunhyang University Hospital) in Seoul, Republic of Korea. This trial was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. An independent ethics committee or Institutional Review Board (IRB) at each study site approved the study protocol (IRB no. SMC 2018-09-073 [Samsung Medical Center], SC19MEDV0003 [Yeouido St. Mary’s Hospital], 2019-01-019 [Kyung Hee University Hospital at Gangdong], and 2018-12-024 [Soonchunhyang University Hospital]). All participants provided written informed consent before eligibility screening.
2. Eligibility
Eligible participants were ambulatory postmenopausal women aged 55 to 70 years with osteoporosis or osteopenia (a T-score of <−1.0 at the total hip, femoral neck, or lumbar spine). To be included, participants had to have 2 or more vertebrae in the L1 through L4 region and at least 1 hip that could be assessed by means of d dual energy X-ray absorptiometry (DXA). Individuals were excluded if they had a history of deep vein thrombosis, pulmonary embolism, retinal vein thrombosis, cerebral infarction, or transient ischemic attack. Furthermore, individuals who had atrial fibrillation, liver cirrhosis, uterine bleeding of unknown cause, symptoms or signs of endometrial cancer, antiphospholipid syndrome, hypercalcemia, hypercalciuria, nephrolithiasis, galactose intolerance, Lapp lactase deficiency, or glucose-galactose malabsorption; pregnant or lactating women, or women whose pregnancy cannot be ruled out; those who had a history of major upper gastrointestinal tract diseases; those who had a history of bisphosphonate treatment (within 2 years prior to screening), hormone replacement therapy or treatment with SERM (within 1 year prior to screening); those who had been taking, within the 3 months prior to screening, vitamin D ≥200 IU daily.
3. Trial procedures
Women were randomly assigned, in a 1:1 ratio, to receive raloxifene/vitamin D combination therapy (raloxifene 60 mg plus cholecalciferol 800 IU) or raloxifene monotherapy at a dose of 60 mg. Randomization was stratified according to the lowest T-score at the total hip, femoral neck, or lumbar spine (T-score ≤−2.5 vs. >−2.5) at each study site. For those in the combination therapy group, one capsule containing raloxifene 60 mg and cholecalciferol 800 IU (Rabone D Cap.; Hanmi Pharm) was taken per oral once daily for 16 weeks. For those in the monotherapy group, one tablet of raloxifene 60 mg (Raloxfen Tab.; Hanmi Pharm) was taken once a day for 16 weeks. All patients were provided once daily calcium carbonate 500 mg during or immediately after a meal. Follow-up visits after baseline were scheduled every 8 weeks for a total of 16 weeks.
The bone mineral density at proximal femur and lumbar spine was assessed by means of DXA (Lunar or Hologic) at baseline. Serum 25(OH)D level was determined by a chemiluminescent microparticle immunoassay with an Alinity i 25-OH Vitamin D Reagent Kit on the Alinity i analyzer (Abbott Laboratories, Abbott Park, IL, USA). Serum concentrations of 25(OH)D, calcium, phosphate, intact parathyroid hormone (iPTH), and bone-turnover markers C-terminal telopeptide of type I collagen (CTX) and bone-specific alkaline phosphatase (BALP) were measured after an 8 hr of overnight fast at the central lab set following Clinical and Laboratory Standards Institute guidelines, at scheduled visits. Complete blood count and routine chemistry including liver function, fasting plasma glucose, blood urea nitrogen, and creatinine were determined at each study site at baseline and week 16.
4. End points
The primary end point was change in serum 25(OH)D level from baseline to 16 weeks after intervention. The key secondary end points were change in serum iPTH value from baseline to weeks 8 and 16, change in serum CTX, BALP, calcium, and phosphate levels from baseline to weeks 8 and 16, change in serum 25(OH)D concentration from baseline to 8 weeks, and drug compliance (defined as the proportion of experimental drugs actually taken by a subject among the total of provided).
5. Statistical analysis
The sample-size calculation was based on the following assumption. Assuming a 6.5 ng/mL difference in change in serum 25(OH)D levels between the study groups and assuming that the standard deviation in each group is the same as 8.74 ng/mL referring to a previous study,[15] 38 subjects are required per group, with a 2-sided α threshold of 0.05 and 90% power. Accounting for expected dropout rate of 20%, 96 were randomly assigned to the control group of raloxifene monotherapy (N=47), or the experimental group of raloxifene/vitamin D combination therapy (N=49).
Baseline demographics and clinical characteristics were analyzed in the randomization set. All efficacy analyses were conducted in the full analysis set (intention-to-treat population consisting of all participants who took one or more doses of assigned drugs with at least one available post-baseline value among those who were randomly assigned). The data with missing values were analyzed according to the last-observation carried forward method. For reporting of safety data, we used the safety analysis set (all participants who received at least one dose of study treatment).
Baseline characteristics between groups were compared using 2-sample t-test or Mann-Whitney test for continuous variables, and Pearson’s χ2 or Fisher’s exact test for categorical variables. Changes at 16 weeks or 8 weeks were compared to the baseline values with the paired t-test or Wilcoxon signed rank test. Changes from baseline to week 16 or week 8 were compared between treatment groups using weighted 2 sample t-test according to the proportion of osteoporosis versus osteopenia. In cases of multiple testing, P-values and confidence intervals (CIs) were corrected by the Bonferroni’s method. Bonferroni’s correction (level=3) was applied in assessing change in serum 25(OH)D level within treatment groups since all pairwise comparisons were performed between baseline, week 8, and week 16. Comparing changes in serum iPTH, CTX, BALP, calcium, and phosphate levels from baseline to week 8 and week 16, and changes in serum 25(OH)D level from baseline to week 8 between groups, Bonferroni’s correction (level=2) was applied since multiple tastings (from baseline to week 8 as well as to week 16) were conducted. The binomial exact CI method was used to estimate the proportion of adverse event (AE). The SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses. Two-sided P-values of less than 0.05 were considered as significant.
6. Subgroup analyses
The primary endpoint was compared in subgroups categorized by the baseline serum 25(OH)D levels (<20 vs. ≥20 ng/mL, and <30 vs. ≥30 ng/mL). Also, the proportion of individuals who exhibited 25(OH)D levels ≥20 ng/mL at week 16 was compared in subgroups of baseline 25(OH)D levels (<20 vs. ≥20 ng/mL).
RESULTS
1. Patient characteristics
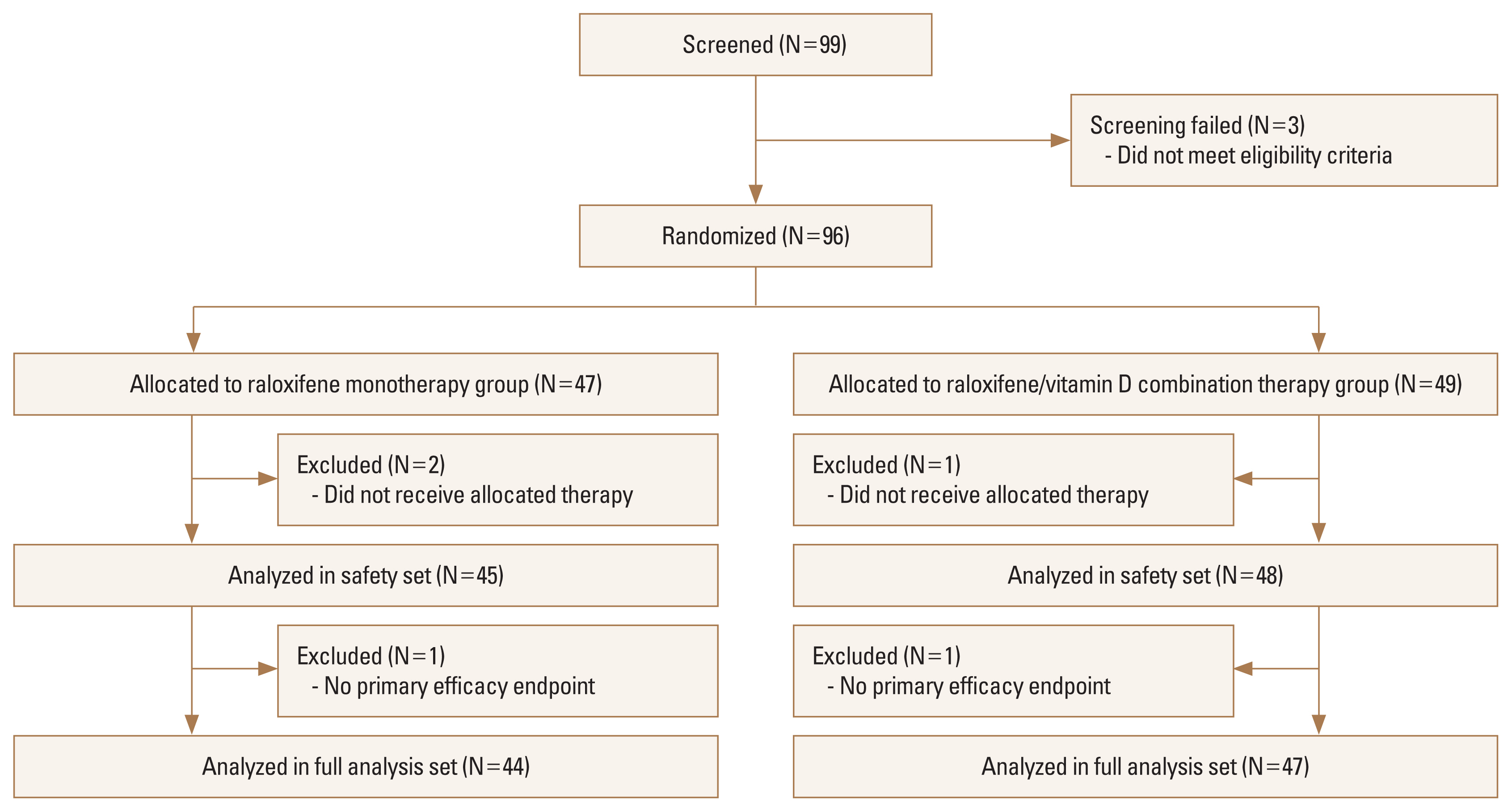
A total of 99 postmenopausal women with osteoporosis or osteopenia underwent screening, and 96 were randomly assigned to raloxifene/vitamin D combination therapy (N=49), or raloxifene monotherapy (N=47) groups. Details of the screening, randomization, and follow-up of the participants are provided in Figure 1. In the full analysis set (intention-to-treat population), 91 participants (47 in raloxifene/vitamin D combination therapy group, and 44 in raloxifene monotherapy group) were included. Demographics, clinical characteristics, and vital signs were similar between the 2 groups at baseline (Table 1). Laboratory results were not significantly different between groups except for the serum iPTH levels. The mean age across the randomization set was 63.3 years (standard deviation [SD], 4.0 years), and mean menopause duration was 12.7 (SD, 5.5) years across the randomization set. Serum 25(OH)D levels were 26.6 (SD, 9.4) ng/mL in raloxifene/vitamin D combination therapy group and 24.0 (SD, 9.9) ng/mL in raloxifene monotherapy group.
2. Primary end point
Mean serum 25(OH)D level increased at week 16 from baseline only in raloxifene/vitamin D combination therapy group (Fig. 2 and Supplementary Table 1). Change in serum 25(OH)D level from baseline to week 16 was significantly higher in raloxifene/vitamin D combination therapy group (2.7±6.5 ng/mL) than in raloxifene monotherapy group (−1.7±6.2 ng/mL) (Table 2).
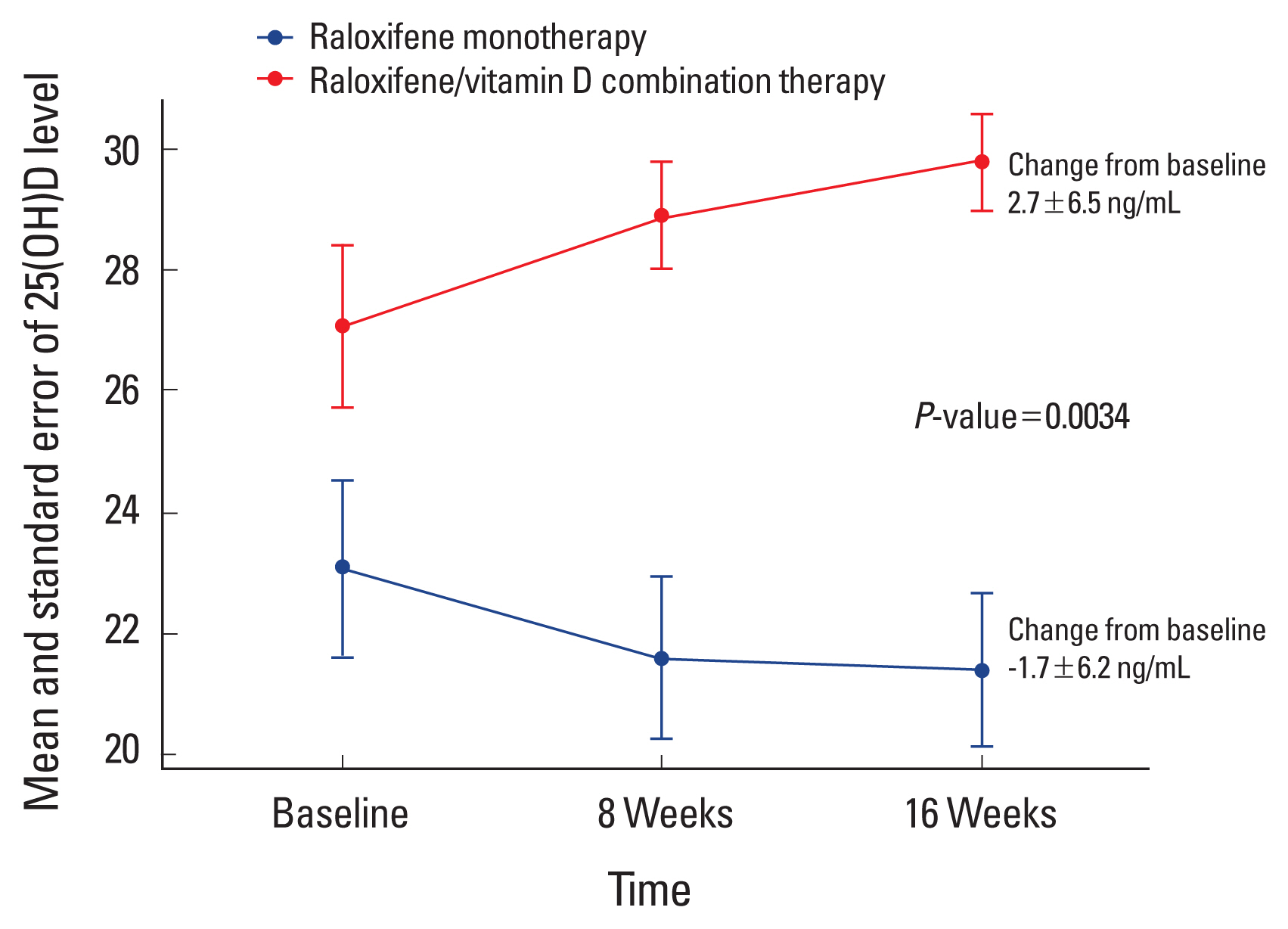
Mean serum 25-hydroxy-vitamin D (25[OH]D) levels according to treatment groups at baseline, week 8, and week 16. Serum 25(OH)D levels were not significantly different between groups at baseline. However, change in serum 25(OH)D level from baseline to week 8 or 16 was significantly higher in raloxifene/vitamin D combination therapy group than in raloxifene monotherapy group. Mean serum 25(OH)D level increased at week 16 from baseline only in raloxifene/vitamin D combination therapy group.
3. Secondary and additional end points
Change in serum 25(OH)D level from baseline to week 8 was significantly higher in raloxifene/vitamin D combination therapy group (1.8±5.8 ng/mL) than in raloxifene monotherapy group (−1.5±4.7 ng/mL) (Supplementary Table 2). Change in serum iPTH value from baseline to weeks 8 and 16, change in serum CTX, BALP, calcium, and phosphate levels from baseline to weeks 8 and 16, and drug compliance for experimental drugs and calcium carbonate were not significantly different between groups (Supplementary Table 2). Drug compliance for experimental drugs were 94.2 (SD, 6.5) % in raloxifene/vitamin D combination therapy group and 94.3 (SD, 5.7) % in raloxifene monotherapy group.
4. Safety end points
The safety analysis set (all participants who received at least one dose of study treatment) included 93 participants (48 in raloxifene/vitamin D combination therapy group, and 45 in raloxifene monotherapy group). Among 15 participants (31.3%) in raloxifene/vitamin D combination therapy group, 23 cases of any AEs were noted, and 11 any AEs were observed among 10 participants (22.2%) in raloxifene monotherapy group. These proportions were not significantly different between groups (Table 3). With respect to the causal relationship with the trial intervention, 6 were possible, and the rest 28 were classified as unlikely among a total of 34 AEs. When analyzed after categorizing the AEs according to System-Organ Class, the proportions and number of AEs were not significantly different between groups, either. There was only one case of severe AE (spondylolisthesis, in raloxifene/vitamin D combination therapy group), which was considered as unlikely to be related to trial intervention, and no action was taken with the study drug in this case (Supplementary Table 3). During the trial, there were 4 AE cases led to the discontinuation or temporary discontinuation of study drugs, and they are also summarized in Supplementary Table 3.
5. Analyses in subgroups categorized by the baseline serum 25(OH)D levels
The increase in serum 25(OH)D concentration in raloxifene/vitamin D combination therapy group compared to raloxifene monotherapy group was more pronounced in individuals with lower categories of baseline 25(OH)D level (Supplementary Table 4). The mean change in serum 25(OH)D level from baseline to week 16 in raloxifene/vitamin D combination therapy group was 10.5±6.1 ng/mL among women with baseline serum 25(OH)D level <20 ng/mL, and 0.6±4.7 ng/mL among women with baseline serum 25(OH)D level ≥20 ng/mL. Among 28 women who exhibited baseline 25(OH)D level of <20 ng/mL, only 3 of 18 (16.7%) individuals in the raloxifene monotherapy group achieved serum 25(OH)D level of ≥20 ng/mL at week 16 whereas all the 10 individuals in the raloxifene/vitamin D combination therapy group showed serum 25(OH)D level of ≥20 ng/mL l at week 16 (Supplementary Table 5).
DISCUSSION
In this open-label, randomized, multicenter clinical trial involving postmenopausal women with osteoporosis or osteopenia, a fixed dose combination of raloxifene 60 mg and vitamin D 800 IU resulted in superior efficacy for increasing serum 25(OH)D levels compared to the raloxifene 60 mg alone. Furthermore, the relative safety and tolerability of combined vitamin D 800 IU added to raloxifene 60 mg were also demonstrated in this trial.
As expected, compared to raloxifene monotherapy, the addition of cholecalciferol 800 IU in a combined capsule of raloxifene/vitamin D for 16 weeks significantly increased serum 25(OH)D level. The significantly higher increase in serum 25(OH)D level in raloxifene/vitamin D combination therapy group compared to raloxifene monotherapy group was observed already at week 8 and persisted at week 16. Therefore, a fixed dose combination capsule of raloxifene 60 mg and vitamin D 800 IU can be an efficient and convenient strategy to improve vitamin D status in addition to the protective effect of raloxifene on bone health among postmenopausal women with osteoporosis or osteopenia.
Appropriate vitamin D status is an essential component of bone health,[8–10] and a prerequisite for optimal response to anti-resorptive agents (including raloxifene) in postmenopausal women.[11] Furthermore, an adequate supply of vitamin D seems to be helpful for preventing falls and fractures and managing osteoporosis in postmenopausal women.[12] Thus, the superior efficacy of raloxifene/vitamin D combination therapy compared to the raloxifene monotherapy for increasing serum 25(OH)D levels might be led to a more favorable effect on bone mineral density and fracture risk, particularly among those with vitamin D inadequacy. Consistently, in a previous randomized trial conducted among postmenopausal Japanese women with osteoporosis or osteopenia and followed for 2 years, supplementation with alfacalcidol to raloxifene therapy showed greater bone-sparing effect than raloxifene-monotherapy.[16]
However, the degree of increase in serum 25(OH)D level in raloxifene/vitamin D combination therapy group in our study was relatively subtle compared to the previous studies that assessed the effect of vitamin D supplementation on serum 25(OH)D concentration.[15,17] Considering that no clinically relevant pharmacokinetic interactions have been shown between raloxifene and cholecalciferol,[18] this is not likely to be related to the influence of raloxifene. Rather, characteristics of the study population including relatively higher baseline serum 25(OH)D level (mean 25.3 ±9.7 ng/mL) might have contributed. In a meta-analysis of randomized trials that evaluated the influence of vitamin D supplementation, high baseline 25(OH)D level was non-significantly associated with lower increases in 25(OH)D concentrations.[17] Also, in a pooled analysis of randomized trials, individuals with the lowest baseline serum 25(OH)D concentration had the highest increase (Δ) after supplementation.[19] Consistently, when the primary endpoint was compared in subgroups categorized by the baseline serum 25(OH)D levels in our study, the increase in serum 25(OH)D concentration in raloxifene/vitamin D combination therapy group compared to raloxifene monotherapy group was more pronounced in individuals with lower baseline 25(OH)D level, and the effect of coadministration of vitamin D was attenuated in those with baseline 25(OH)D level ≥30 ng/mL (Supplementary Table 4). The mean change in serum 25(OH)D level from baseline to week 16 was as high as 10.5 ng/mL in raloxifene/vitamin D combination therapy group among women with baseline serum 25(OH)D level <20 ng/mL.
In the current trial, the overall safety of raloxifene/vitamin D combination therapy was similar to that of raloxifene monotherapy. The only one case of severe AE was spondylolisthesis in raloxifene/vitamin D combination therapy group and was unlikely to be related to trial intervention. Also, in a previous randomized phase 1 trial that explored the pharmacokinetic interaction between raloxifene and cholecalciferol in healthy male volunteers, coadministration of raloxifene and cholecalciferol did not affect the individual pharmacokinetics of each drug, and no serious or unexpected AEs were detected, suggesting that raloxifene and cholecalciferol have no clinically relevant pharmacokinetic drug-drug interactions with concurrent administration.[18]
Several limitations of this study should be acknowledged. First, our study population was restricted to Korean postmenopausal women aged 55 to 70 years with osteoporosis or osteopenia. Therefore, effects beyond those in the studied population cannot be appropriately inferred, and extrapolation of our findings to other populations such as those with different ethnicity should be cautious. Second, 16 weeks might not be enough to explore long-term sustainable efficacy and safety, and additional studies with longer-term follow-up may be needed. Third, effects on bone mineral density or fracture risk were not compared. Since only effects on vitamin D status were evaluated, the influence of treatment on bone health cannot be determined with the current study. Particularly among women with already sufficient vitamin D status at baseline, in whom vitamin D supplementation with a combined capsule of raloxifene/vitamin D showed only modest additional effects on vitamin D status, potential benefits of treatment on bone health cannot be guaranteed. Fourth, our study participants might not represent the general postmenopausal women with osteoporosis or osteopenia. Our study population is considered as highly motivated women with high interest in their own health as we enrolled otherwise healthy women who visited university-affiliated hospitals for the evaluation or management of osteoporosis or osteopenia and voluntarily participated in the clinical trial. This might be related to the relatively high baseline serum 25(OH)D level in our study.
To summarize, a 16-week treatment with a fixed dose combination of raloxifene 60 mg and vitamin D 800 IU provided better efficacy in elevating serum 25(OH)D levels compared with raloxifene 60 mg alone without vitamin D supplementation among postmenopausal women with osteoporosis or osteopenia. In addition, the safety of the combination capsule of raloxifene/vitamin D 800 IU was comparable to that of raloxifene alone. Taken together, a fixed dose combination of raloxifene 60 mg and vitamin D 800 IU may be efficaciously and safely used for postmenopausal women with osteoporosis or osteopenia to improve serum 25(OH)D levels.
Supplementary Information
Notes
Funding
This study was supported by research grants from Hanmi Pharmaceutical Co., Ltd.
Ethics approval and consent to participate
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by an independent ethics committee or Institutional Review Board (IRB) at each study center (IRB No. SMC 2018-09-073 [Samsung Medical Center], SC19MEDV0003 [Yeouido St. Mary’s Hospital], 2019-01-019 [Kyung Hee University Hospital at Gangdong], and 2018-12-024 [Soonchunhyang University Hospital]). All participants provided written informed consent before eligibility screening.
Conflict of interest
No potential conflict of interest relevant to this article was reported.