Inhibitory Effect of Rosae Multiflorae Fructus Extracts on the Receptor Activator of NF-κB Ligand-Induced Osteoclastogenesis through Modulation of P38- and Ca2+-Mediated Nuclear Factor of Activated T-Cells Cytoplasmic 1 Expression
Article information
Abstract
Background
Rosae Multiflorae fructus (RMF), known to have anti-inflammatory and antioxidant properties, has been used as a traditional remedy for inflammatory diseases such as arthritis in Eastern Asia. However, its effect on osteoclasts, which play a crucial role in resorptive inflammatory bone diseases, is yet to be elucidated.
Methods
The effect of extract of RMF (RMF-E) on receptor activator of nuclear factor-κB ligand (RANKL)-mediated osteoclastogenesis was examined by tartrate-resistant acid phosphatase (TRAP) staining, real-time polymerase chain reaction and western blot analysis. In addition, RANKL-induced Ca2+-oscillation was also investigated.
Results
RMF-E remarkably inhibited TRAP+-osteoclast and resorptive pit formation in a dose-dependent manner. In addition, the expression of c-Fos and nuclear factor of activated T-cells cytoplasmic, known as pivotal transcription factors for osteoclast formation in vitro and in vivo, and that of the osteoclast differentiation markers such as Acp5, Oscar, CtsK, Atp6v0d2, Tm7sf4, and Nfatc1 were significantly decreased by RMF-E treatment during osteoclastogenesis. The inhibitory effect of RMF-E on RANKL-induced osteoclastogenesis was caused by the suppression of p38 mitogen-activated protein kinase activation, and RANKL-induced Ca2+-oscillation removal via inactivation of Bruton's tyrosine kinase (BTK), and subsequently phospholipase C-γ2.
Conclusions
RMF-E negatively regulates osteoclast differentiation and formation. These findings suggest the possibility of RMF-E as a traditional therapeutic agent against osteoclast-related bone disorders such as osteoporosis, rheumatoid arthritis, and periodontitis.
INTRODUCTION
Osteoclasts, which work to resorb the bone matrix, are responsible for maintaining bone homeostasis, and remodeling with osteoblasts, which make bone matrix. Osteoclasts originate from the monocyte/macrophage lineage, and are differentiated by the stimulation of the indispensable osteoclast differentiation cytokine receptor activator of nuclear factor-κB ligand (RANKL).[1] Complicated and delicate intracellular signaling pathways induced by the interaction of RANKL and its receptor molecule; receptor activator of nuclear factor-κB (RANK), have been identified.[23] RANKL to RANK interaction activates nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPK; extracellular signal-regulated kinase [ERK], c-JUN N-terminal kinase [JNK], and p38), through tumor necrosis factor receptor-associated factor 6-dependent signaling, followed by c-Fos upregulation. In another pathway known as co-stimulatory signaling, RANKL/RANK binding triggers the activation of immunoreceptor tyrosine-based activation motif (ITAM)-harboring adaptors, Fc receptor common γ subunit (FcRγ), or DNAX-activating protein of kDa 12 (DAP12), subsequently inducing the activation of Bruton's tyrosine kinase (BTK) and phospholipase C-γ2 (PLCγ2).[2456] PLCγ2 activation produces inositol triphosphate (IP3) which binds to IP3 receptors, from phosphatidylinositol-4,5-bisphosphate, followed by generating Ca2+-oscillation which is important for osteoclast differentiation, via Ca2+ release from the endoplasmic reticulum.[478] Both signaling pathways consequently induce the expression and activation of nuclear factor-activated T cells c1 (NFATc1), known to be the most important transcription factor for osteoclast differentiation, activation, and survival, both in vitro and in vivo.[9]
Rosae Multiflorae fructus (RMF) is the dried fruit of Rosa multiflora Thunberg (‘Youngsil’ in Korean, ‘Yingshi’ in Chinese, and ‘Eijitsu’ in Japanese), known as Multiflora rose; originated from Korea, China, and Japan, and is known to have potential anti-inflammatory, pain-relief, and antioxidant properties.[1011121314] RMF shows no critical toxicity, and has been used as tea, jam, and juice.[1015] As a herbal remedy, RMF has been used traditionally for various diseases, including inflammatory disorders, cold, flu, edema, beriberi, and chronic pain.[121316] Recently, in addition to its anti-inflammatory and analgesic effects in rodent models,[17] several reports indicate that RMF exerts its effects on allergic inflammatory diseases like asthma, food allergy accompanying anaphylaxis, and allergic rhinitis (AR), via functional modulation of Th2 and mast cells, including Th2 cytokine production and histamine release, respectively.[1218] Additionally, previous reports have shown the obvious inhibitory effects of herbal formula (RL) containing RMF and Lonicerae Japonica Flos, on Toll-like receptor (TLR) signaling and collagen-induced arthritis (CIA). RL reportedly suppresses IκB-α and MAPK activation by inhibiting the interleukin (IL)-1 receptor associated kinase/transforming growth factor-β activated kinase 1 and TBK1/interferon regulatory factor 3 (IRF3) pathways, resulting in inhibitory modulation of transcriptional factors such as activator protein-1 (AP-1), NF-κB, and IRF3, and reduction of various pro-inflammatory cytokine and chemokine production in lipopolysaccharide-stimulated RAW264.7 and THP-1 cells.[1019] Moreover, RL also reportedly inhibited TLR-4 signaling, and significantly improved clinical symptoms of CIA rats, including the suppression of bone erosion and osteophyte formation in joints.[11]
Although several reports on its anti-inflammatory effects exist, the specific effects of RMF on osteoclastogenesis remain unknown. Here, we provide biological data regarding the inhibitory effects of RMF extract (RMF-E), via modulation of intracellular p38- and Ca2+-signaling, on RANKL-induced osteoclastogenesis.
METHODS
1. Experimental animals and reagents
The C57BL/6N mice used in this study were purchased from Orient Bio Inc. (Seongnam, Korea). All mouse studies were performed following the protocol (WKU16-87) approved by the Animal Care and Use Committee of Wonkwang University.
Cell culture agents, including media, fetal bovine serum (FBS), and supplements, were purchased from Hyclone (Pierce, Rockford, IL, USA). Soluble recombinant mouse RANKL and recombinant human macrophage colony-stimulating factor (M-CSF) were supplied by T Kim (KIOM, Daejeon, Korea). Anti-NFATc1 and anti-c-Fos antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and anti-actin antibody from Sigma-Aldrich (St. Louis, MO, USA). Antibodies against other proteins used in this study (phospho-ERK [p-ERK; Thr202/Tyr204], ERK, phospho-JNK [Thr183/Tyr185], JNK, p-p38 [Thr180/Tyr182], p-38, phospho-IκBα [Ser32], IκBα, phospho-BTK [Ser180], BTK, phospho-PLCγ2 [Tyr759], and PLCγ2) were purchased from Cell Signaling Technology (Danvers, MA, USA).
2. Preparation of ethanol RMF-E
RMF was purchased in May 2012 from the University Oriental Herbal Drugstore (Iksan, Korea). A voucher specimen (No. NNMBS-2012-046) was deposited at the Herbarium of the College of Pharmacy, Wonkwang University (Iksan, Korea). Dried and pulverized RMF (50 g) was extracted with hot 70% aqueous ethanol (EtOH) for 2 hr, and filtered with filter paper. The filtrate was evaporated in vacuo, to produce a 70% EtOH extract (16.28 g). The extract was then suspended with distilled water (100 mL), followed by filtration, to obtain the precipitate (1.13 g; NNMBS-2012-046). Finally, RMF-E was dissolved in dimethyl sulfoxide and used in all experiments.
3. Cell viability assay
The EZ-Cytox enhanced cell viability assay kit (ItsBio, Seoul, Korea) was purchased for performing the cell viability assay, following the manufacturer's instructions. Briefly, bone marrow (BM) cells from the tibiae and femora of 6 to 8 week-old C57BL/6N mice were cultured in α-minimal essential medium supplemented with 10% FBS and M-CSF (30 ng/mL) for 3 days. The adherent cells, considered as BM-derived macrophage (BMMs), were plated in 96-well culture plates at a density of 1×104 cells/well, with various RMF-E (0, 5, 10, 20, 30, and 40 µg/mL) concentrations, and cultured for 1 day with M-CSF (30 ng/mL), or 4 days with M-CSF under 30 µg/mL RMF-E treatment. The EZ-Cytox reagent was added to cultured cells for 4 hr at 37℃, following the manufacturer's instructions. OD was measured at 450 nm, using a Sunrise™ enzyme-linked immunoassay (ELISA) plate reader (Tecan, Crailsheim, Germany).
4. Assay of in vitro osteoclast differentiation, actin ring, and pit formation
BMM preparation and osteoclast formation were done as previously described.[20] To assess osteoclastogenesis, BMMs were cultured in the presence of M-CSF (30 ng/mL) and RANKL (100 ng/mL), at various concentrations of RMF-E (0, 2, 5, 10, 20, and 30 µg/mL), for 4 days. Media containing M-CSF, RANKL, and RME-F was replaced on day 3. Cells were fixed with 10% formalin, and permeabilized with EtOH/Acetone (1:1). Cells were then stained with rhodamine-phalloidin from Molecular Probes (Eugene, OR, USA), to label the F-actin ring, sequentially followed by tartrate-resistant acid phosphatase (TRAP) solution assay and TRAP staining, as previously described.[21] The F-actin ring was measured under a fluorescent inverted microscope from Leica Microsystems Ltd. (Wetzlar, Germany). Total TRAP activity using p-nitrophenyl phosphate from Sigma-Aldrich, as substrate, was measured at an absorbance of 405 nm, and TRAP-positive multinuclear cells (TRAP+-MNCs) containing ≥3 nuclei were counted as osteoclasts. To measure bone resorption activity, BMMs were cultured in a Cosmo Bio 48-well bone resorption assay plate (Oriental Yeast Co., Ltd., Tokyo, Japan) under M-CSF and RANKL treatment with/without RMF-E (20 µg/mL) for 7 days, and then cleared using bleach. The resorption pit area was analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA; NIH 1.52).
5. Real-time quantitative polymerase chain reaction
BMMs were cultured under M-CSF (30 ng/mL) and RANKL (100 ng/mL) for 4 days with/without RMF-E (20 µg/mL). One microgram of total RNA, extracted using the Trizol reagent from Invitrogen (Carlsbad, CA, USA) at the indicated time points, was transcribed to first strand cDNA with random primers using Maxima reverse transcriptase from Thermo Scientific (Seoul, Korea), following the manufacturer's direction. Real-time polymerase chain reaction (PCR) was performed using the VeriQuest SYBR Green qPCR Master mix from Affymetrix (Santa Clara, CA, USA), and StepOnePlus™ Real-Time PCR Systems from Applied Biosystems (Forster City, CA, USA). All results were normalized to glyceraldehyde 3-phosphate dehydrogenase (Gapdh). The primers used in this study were described in Table 1.
6. Western blot analyses
BMMs were cultured with M-CSF (30 ng/mL) and RANKL (100 ng/mL) in the presence or absence of RMF-E (20 µg/mL), for specific time points. In some experiments, RMF-E (20 µg/mL) was pretreated in BMMs supplemented with M-CSF (30 ng/mL) for 2 hr, and the cells stimulated with RANKL (100 ng/mL). To prepare protein lysate, the cells washed with cold phosphate buffered saline were lysed in radioimmunoprecipitation assay buffer (25 mM Tris-HCl, pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate [SDS]) containing 1 mM phenylmethylsulfonyl fluoride, protease-inhibitor cocktail from Roche (Mannheim, Germany), and phosphatase inhibitor tablets from Thermo Scientific, and then the supernatants were collected by centrifugation at 14,000×g for 10 min at 4℃. Thirty micrograms of total lysate were subjected to SDS-polyacrylamide gel electrophoresis (PAGE), and then transferred to Amersham Hybond-P PVDF membranes from GE-Healthcare Life Science (Amersham, UK). Western blot analyses were sequentially performed with 1:1,000 dilutions of the primary antibody and 1:5,000 dilutions of HRP-conjugated IgG, as secondary antibody in TBST (TBS; 50 mM Tris-HCl, pH 7.6, 150 mM NaCl, and 0.1% Tween-20). The enhanced chemiluminescence detection system from Thermo Scientific was used to detect immunoreactive proteins, following the manufacturer's instructions. The quantification of detected proteins was performed using ImageJ software.
7. Measurement of Intracellular Ca2+ concentration ([Ca2+]i)
[Ca2+]i was measured using the fluorescence Ca2+ indicator (Fura-2, AM), as previously described.[22] Briefly, BMMs seeded on coverslips were treated with RANKL (100 µg/mL) to induce osteoclast differentiation and maintained for additional 24 hr. After incubation, Fura-2 (5µM) was loaded into cell culture for 50 min at 37℃. Prior to [Ca2+]i measurement, unloaded fura-2 was washed out by continuous perfusion of N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)-buffered medium containing 10 mM HEPES, pH 7.4, 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 10 mM glucose. RMF-E (20 µg/mL) diluted in HEPES-buffered medium was acutely treated on cells for specific times. Fura-2 inside cells was excited with dual wavelengths (340 and 380 nm), and the emitted wavelength (510 nm) was captured using a CCD camera. Captured images were digitized and presented as F340/380 ratio. MetaFluor software from the Molecular Devices Corporation (Downingtown, PA, USA), was used to analyze captured images.
8. Statistical Analyses
Data were analyzed using the Student's t-test, and are presented as mean±standard deviation. A P value of less than 0.05 was considered statistically significant. All experiments were repeated at least twice, and representative data are shown.
RESULTS
1. Analyses of RMF-E effect on cell viability
Prior to RMF-E experimental use, its cytotoxicity on osteoclast precursors had been verified. When BMMs were cultured with various RMF-E concentrations, no cell cytotoxicity was detected all tested concentrations, up to 40 µg/mL RMF-E (Fig. 1A). In addition, BMMs were treated with 30 µg/mL RMF-E for 4 days, with daily cell viability measurements. No significant difference was observed between the daily cell viabilities of the control (0 µg/mL) and RMF-E-treated (30 µg/mL) cells (Fig. 1B). Therefore, following these cytotoxicity results, 20 µg/mL RMF-E was selected for most experiments in this study.
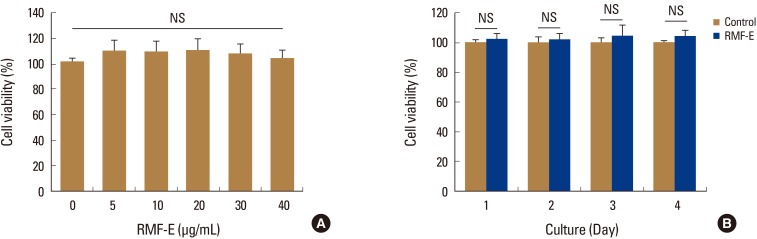
Effects of Rosae Multiflorae fructus extract (RMF-E) on cell viability. (A) Bone marrow-derived macrophages (BMMs) were cultured with the indicated concentrations of RMF-E, in the presence of macrophage colony-stimulating factor (M-CSF) (30 ng/mL) for 1 day. (B) BMMs were cultured with/without 30 µg/mL RMF-E, in the presence of M-CSF, for 4 days. Cell viability was measured as described in the materials and methods. Data are presented as the mean±standard deviation of 3 independent experiments. NS, not significant.
2. Inhibitory effect of RMF-E on osteoclast differentiation and resorption activity
Initially, we investigated the effect of RMF-E on osteoclast differentiation and formation. BMMs were treated with various concentrations of RMF-E in the presence of M-CSF and RANKL for 4 days. The TRAP staining and TRAP solution assay was performed to measure osteoclast differentiation and formation. As shown in Figure 2A and B, RMF-E dramatically inhibited TRAP+-MNCs containing ≥3 nuclei and ≥100 µm in diameter (counted as mature osteoclasts), in a dose-dependent manner. The decrease in TRAP+-MNCs formation commenced in 5 µg/mL RMF-E, and rarely formed in 30 µg/mL RMF-E. Moreover, total TRAP activity from TRAP+-mono-, di-, and multi-nuclear osteoclasts, was also significantly dose-dependently diminished by RMF-E treatment (Fig. 2C). To assess if RMF-E affects osteoclast bone resorbing activity, we measured in vitro F-actin ring and pit formation in osteoclast cultured with/without RMF-E treatment. The F-actin ring structure, which is required for bone matrix resorption in vitro and in vivo, was rarely formed in RMF-E-treated osteoclasts, as opposed to the untreated control (Fig. 2D). Eventually, RMF-E-treated osteoclasts showed a dramatically decreased pit formation, compared with the untreated control (Fig. 2E). The suppression of resorption activity via F-actin ring and pit formation is attributable to decreased TRAP+-MNC formation in RMF-E-treated osteoclasts. These data demonstrate that RMF-E conveniently represses osteoclast differentiation and activity.

Effect of Rosae Multiflorae fructus extract (RMF-E) on osteoclast differentiation and resorption activity. Bone marrow-derived macrophages were cultured with the indicated concentrations of RMF-E, under receptor activator of nuclear factor-κB ligand (100 ng/mL) and macrophage-colony stimulating factor (30 ng/mL) treatment, for 4 days. (A) Osteoclasts were fixed and stained for tartrate-resistant acid phosphatase (TRAP). (B) TRAP+-multinuclear cells with ≥3 nuclei were counted as mature osteoclasts. (C) Total TRAP activity from TRAP+-mono-, di-, and multi-nuclear cells were measured at an absorbance of 405 nm (A405 nm). (D) F-actin rings were stained with rhodamine-phalloidin in osteoclasts treated with/without RMF-E (20 µg/mL). (E) The resorption activity of osteoclasts treated with/without RMF-E (20 µg/mL) was measured by pit formation on hydroxyapatite-coated plates, after 7 days. Data are expressed as the mean±standard deviation and are representative of 3 independent experiments. *P<0.05, **P<0.01, and ***P<0.001 vs. the non-treated control (NC, 0 µg/mL RMF-E) (Scale bar=200 µm). NS, not significant.
3. Inhibitory effect of RMF-E on the expression of osteoclast differentiation factors
To verify RMF-E inhibitory effect on osteoclast differentiation at the molecular level, the expression of osteoclast differentiation marker genes (Acp5, Oscar, CtsK, Atp6v0d2, Tm7sf4, and Nfatc1) and osteoclast differentiation critical transcription factors (c-Fos and NFATc1) was measured using real-time PCR and/or immunoblot analyses, during RANKL-mediated osteoclastogenesis. The expression of all tested marker genes, including Nfatc1, was significantly inhibited in RMF-E-treated osteoclasts (Fig. 3A). Additionally, c-Fos and NFATc1 expression were considerably suppressed by RMF-E treatment (approximately 35% and 55%, respectively), compared with those of the control cells (Fig. 3B, C). These results support the previous results (Fig. 2) regarding RMF-E inhibitory effect on RANKL-induced osteoclast differentiation and formation.
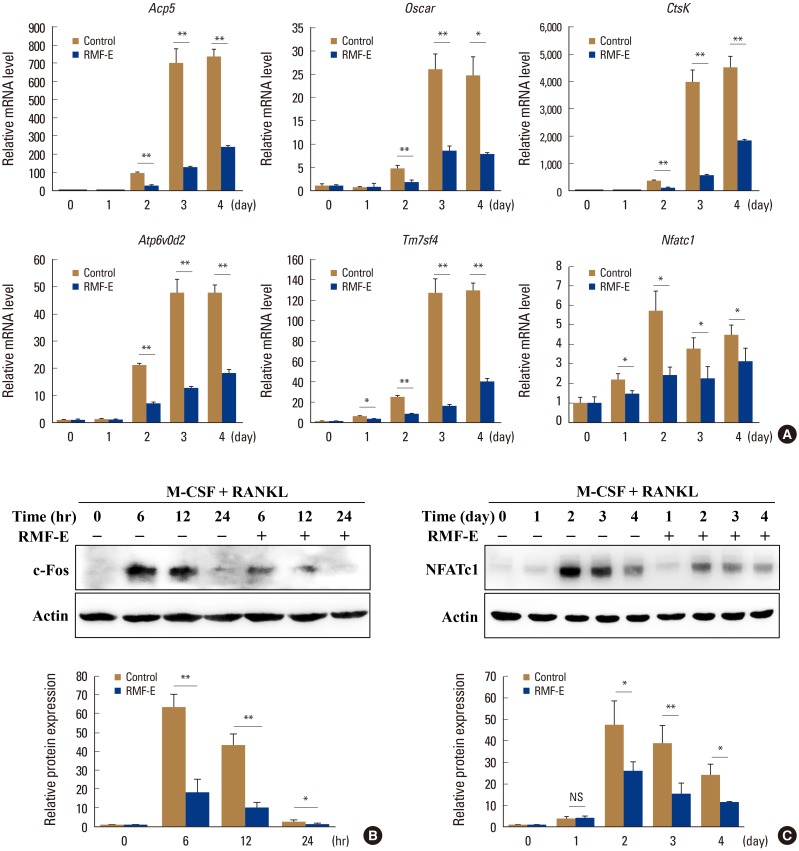
Effects of Rosae Multiflorae fructus extract (RMF-E) on the expression of osteoclast differentiation marker genes and transcription factors. Bone marrow-derived macrophages were cultured with receptor activator of nuclear factor-κB ligand (RANKL) (100 ng/mL) and macrophage colony-stimulating (M-CSF) treatment in the presence or absence of RMF-E (20 µg/mL) for 4 days or the indicated times (for c-Fos). (A) Osteoclast differentiation marker gene expression was examined by real-time polymerase chain reaction. Messenger RNA (mRNA) levels were normalized with Gapdh and expressed as fold change of mRNA level. (B, C) Whole lysate (30 µg) was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and analyzed by immunoblotting. c-Fos (B) and nuclear factor-activated T cells c1 (NFATc1) (C) expression were detected using the anti-c-Fos and NFATc1 antibody, respectively. Fold change normalized by actin is presented in the lower panel. Data are expressed as the mean±standard deviation and are representative of 3 independent experiments. *P<0.05 and **P<0.01 vs. the control group (0 µg/mL RMF-E).
4. Inhibitory mechanism of RMF-E on RANKL-induced osteoclastogenesis
As observed, RMF-E suppressed the expression of c-Fos and NFATc1, pivotal osteoclastogenesis transcription factors (Fig. 3). Therefore, we attempted to examine how RMF-E down-regulates c-Fos and NFATc1 expression, underlying osteoclast differentiation and formation. First, the activation of NF-κB and MAPKs, the main signaling pathway induced by the RANKL/RANK interaction, were examined. BMMs were pre-cultured with/without RMF-E (20 µg/mL) under M-CSF treatment, and RANKL-induced MAPKs and IκBα activation in measured using Western blot analyses. The activation of p38 looked like a little repressed, but overall the activation of IκBα and MAPKs seem not affected by RMF-F treatment (Fig. 4A).
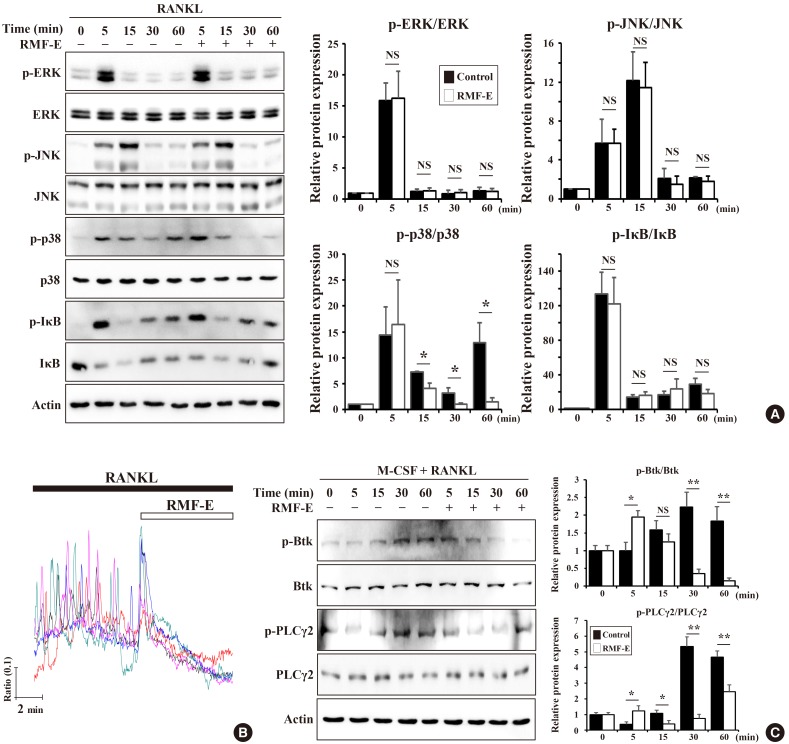
Effects of Rosae Multiflorae fructus extract (RMF-E) on receptor activator of nuclear factor-κB ligand (RANKL)-induced intracellular signaling. Bone marrow-derived macrophages (BMMs) were pretreated with/without RMF-E (20 µg/mL) for 2 hr in the presence of macrophage colony-stimulating factor (M-CSF) (30 ng/mL), and then RANKL (100 ng/mL)-treated, to stimulate intracellular signaling, at indicated times. Lysate (30 µg) was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and analyzed by immunoblotting. (A) The activation of mitogen-activated protein kinases (extracellular signal-regulated kinase [ERK], c-JUN N-terminal kinase [JNK], and p38) and IκBα was examined using their respective antibodies. (B) Twenty four-hr RANKL-stimulated BMMs were acutely treated with RMF-E (20 µg/mL). RANKL-stimulated Ca2+-oscillation by was measured using the fluorescence Ca2+ indicator (Fura-2, AM). (C) Bruton's tyrosine kinase (BTK) and phospholipase C-γ2 (PLCγ2) activation were detected using the anti p-BTK/BTK and p-PLCγ2/PLCγ2 antibody, respectively. Fold change normalized by their non-phosphorylated proteins is presented in the right panel. Data are expressed as mean±standard deviation and are representative of 3 independent experiments. *P<0.05 and **P<0.01 vs. the control group (0 µg/mL RMF-E).
The RANKL/RANK interaction is also capable of stimulating Ca2+ signaling via adaptor protein containing ITAM such as DAP12 and FcRγ, followed by modulation of NFATc1 expression and activation. Next, we examined if RMF-E affects RANKL-induced Ca2+-oscillation. BMMs cultured under RANKL stimulation were acutely treated with RMF-F. Before RMF-E treatment, RANKL-stimulated cells exhibited typical Ca2+-oscillation. However, as soon as RMF-F treatment commenced, intracellular Ca2+ concentration frequency immediately disappeared (Fig. 4B). We then examined if RMF-E affects RANKL-stimulated BTK and PLCγ activation responsible for intracellular Ca2+-oscillation in upstream RANKL-signaling. BTK and PLCγ were normally activated by RANKL stimulation. Although RMF-E pretreated cells showed a slight increase at 5 min, BTK and PLCγ2 phosphorylation were gradually suppressed in RMF-E pretreated cells, compared with those of untreated control cells (Fig. 4C).
Collectively, these data demonstrate that RMF-E regulates MAPKs, specifically p38, as well as BTK/PLCγ2 activation and RANKL-induced Ca2+-oscillation, which are all important factors for c-Fos and NFATc1 induction in RANKL-stimulated osteoclast differentiation and formation (Fig. 5).
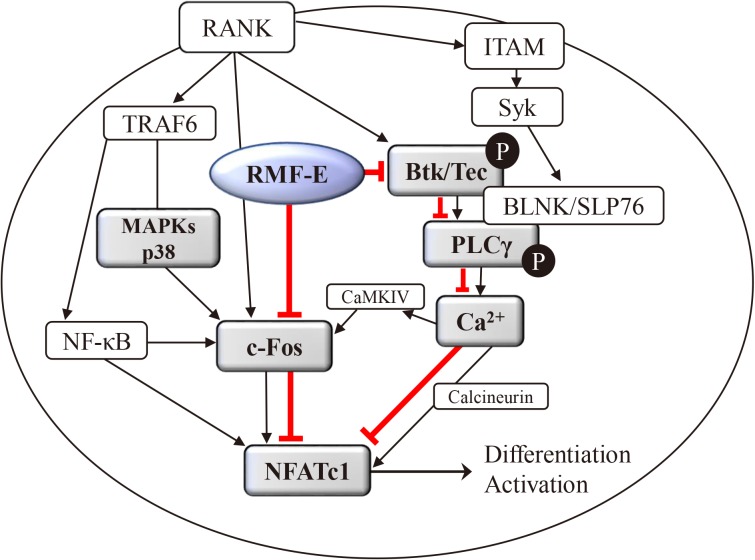
Schematic diagram of the effect of Rosae Multiflorae fructus extract (RMF-E) on receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclastogenesis. The RANKL/RANK interaction may lead to mitogen-activated protein kinases (MAPKs) activation, followed by induction of c-Fos expression, and it also leads to Bruton's tyrosine kinase (BTK) and phospholipase C-γ2 (PLCγ2) activation inducing calcium signaling, followed by induction of nuclear factor-activated T cells c1 (NFATc1) expression and activation. RMF-E may inhibit NFATc1 induction via suppression of c-Fos and modulation of the BTK/PLCγ signaling pathways regulating Ca2+ signaling, resulting in the inhibition of RANKL-induced osteoclastogenesis. The red line indicates the inhibition pathway of RMF-E. TRAF6, tumor necrosis factor receptor associated factor 6; ITAM, immunoreceptor tyrosine-based activation motif.
DISCUSSION
For several decades, a good number of studies have demonstrated the medicinal effectiveness of extracts or constituents from various plants used as traditional medicines against resorptive bone diseases, including osteoporosis. [23] Although their potential anti-bone loss effects have been suggested, only some of these plants have been appropriately examined for their physiological and pharmacological properties, and their action mechanism. The presented medicinal plants have been reported to modulate the differentiation of osteoclasts, osteoblasts, or both, by diverse effects and action mechanisms.[23] Although molecular action mechanisms of medicinal plant extracts have been known and found responsible for the regulation of osteoclast differentiation, intracellular signaling for the activation of MAPKs as well as NF-κB is dominantly modulated by medicinal plants, followed by inhibition of c-Fos and NFATc1, known as critical osteoclastogenic transcription factors.[24252627]
In Eastern Asian countries like Korea and China, RMF has been traditionally used to calm inflammatory disorders such as arthritis.[13] In western countries including both Europe and the United States, the rosehips of Rosa canina, another wild rose like multiflora rose, have also been clinically used as osteoarthritis remedies.[2829] Although RMF has been linked to inflammatory bone disease treatment, the precise underlying mechanism of its effect on bone cells, especially osteoclasts, remains unelucidated. In this study, we investigated the effect of RMF-E on RANKL-mediated osteoclast differentiation and activation. RMF-E inhibited RANKL-induced TRAP+-mature osteoclast formation and bone resorption activity, accompanied by repression of osteoclast differentiation markers, in a dose-dependent manner (Fig. 2, 3A). In addition, c-Fos and NFATc1 expression were decreased by RMF-E treatment during RANKL-mediated osteoclastogenesis (Fig. 3B). These RMF-E inhibitory effects appeared to be partially caused by downregulation of p38 MAPK activation.
In terms of RANKL-RANK interaction-induced osteoclastogenesis, the Ca2+signaling pathway via FcRγ-DAP12/BTK/PLCγ signaling, also affects the expression of transcription factors such as c-Fos and NFATc1.[468] Our previous reports already showed that the inhibitory effects of the EtOH extracts of some herbal plants (Chrysanthemum zawadskii Herbich var. and Glechoma hederacea) on RANKL-induced osteoclastogenesis was due to Ca2+ signaling pathway modulation, followed by downregulation of NFATc1.[2230] In the present study, RMF-E also exerted its inhibitory effect by modifying Ca2+ signaling via BTK/PLCγ2 pathway inactivation (Fig. 4B, C). Overall, it appears RMF-E can regulate the canonical signal pathway by MAPK activation, and the co-stimulatory signal pathway by Ca2+-oscillation, during RANKL-mediated osteoclastogenesis. Although many other signaling pathways are capable of regulating osteoclastogenesis, those related to reactive oxygen species production and inflammation, and the role of RMF-E in anti-oxidant and anti-inflammation activities, are not mentioned in this study. There is a need to investigate these functional RMF-E activities, to enable a detailed understanding of its effect on RANKL-mediated osteoclastogenesis.
RMF methanol extracts have been reported to have many constituents, including quercetin derivatives (such as quercitrin and multinoside A), kaempferol derivatives (such as multiflorins A and B), carotene, vitamin E, essential fatty acids, and minerals.[1331] In view of osteoclastogenesis, Wattel et al. [32] had presented quercetin to have an inhibitory effect on RANKL-mediated osteoclastogenesis in Raw 264.7 and peripheral blood momocytic cells, via modulation of NF-κB and AP-1 activation. Kaempferol was also shown to have inhibitory effect on RANKL-mediated and IL-1β-stimulated osteoclastogenesis, via inactivation of MAPKs (ERK, JNK, and p38), and suppression of c-Fos and NFATc1.[33] These results are almost consistent with those of this study. However, till date, no reports exist on the known RMF-E constituent(s) related to the inhibition of BTK/PLCγ/Ca2+-oscillation signaling pathway. It is therefore vital to investigate which RMF-E constituent is responsible for modulating Ca2+ signaling in RANKL-induced osteoclastogenesis. For future studies, RMF-E component analyses and precise action mechanism assessment, including Ca2+ signaling, of its constituents, are needed to understand the effect of RMF-E on RANKL-mediated osteoclastogenesis. Moreover, its in vivo studies using animal models for bone-resorption associated bone diseases such as osteoporosis, rheumatoid arthritis, and periodontitis, will most likely provide more in-depth knowledge for comprehending the delicate effect of RMF-E on RANKL-mediated osteoclastogenesis and bone metabolism.
Acknowledgments
This paper was supported by Wonkwang University in 2017.
Notes
Ethics approval and consent to participate: All studies involving animals were approved by the Animal Care and Use Committee of Wonkwang University
Conflict of interest: No potential conflict of interest relevant to this article was reported.

