Risk of Osteoporotic Fracture in Patients with Breast Cancer: Meta-Analysis
Article information
Abstract
Background
The fracture risk induced by anti-estrogen therapy in patients with breast cancer remains controversial. The aim of this study was to perform a meta-analysis and systematic review to evaluate the risk of osteoporotic fracture in patients with breast cancer.
Methods
A systematic search was performed to identify studies that included any osteoporotic fracture (hip fracture and vertebral fracture) in patients breast cancer. Main outcome measures were occurrence and risk of osteoporotic fractures including hip and vertebral fractures in patients and controls.
Results
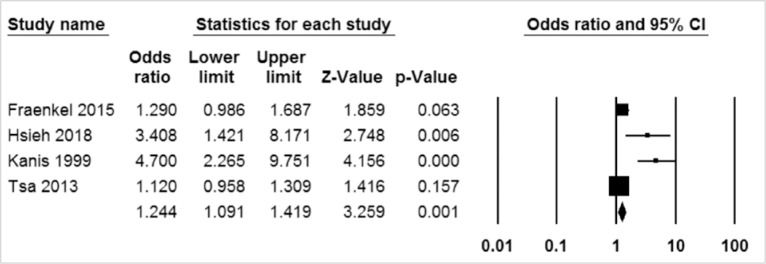
A systematic search yielded a total of 4 studies that included osteoporotic fracture outcomes in patients with breast cancer. Meta-analysis showed a higher risk of osteoporotic fracture in patients with breast cancer. Analysis of these 4 studies involving a total of 127,722 (23,821 cases and 103,901 controls) patients showed that the incidence of osteoporotic fractures was higher in the breast cancer group than in the control group. The pooled estimate of crude relative risk for osteoporotic fracture was 1.35 (95% confidence interval, 1.29–1.42; P<0.001).
Conclusions
Although studies were limited by a small number, results suggested a possible association between anti-estrogen therapy and increased risk of osteoporotic fractures in patients with breast cancer.
INTRODUCTION
Breast cancer is the most commonly diagnosed cancer in women worldwide.[12] Breast cancer has been considered as an estrogen-hormone dependent cancer because its occurrence and recurrence depend on the status of hormone and hormone receptor in cancer cells.[3] Therefore, anti-estrogen therapy using tamoxifen or aromatase inhibitors (AIs) has been the gold standard adjuvant endocrine therapy to treat hormone receptor-positive breast cancer [45] and to control recurrent or metastatic disease.[6]
Anti-estrogen therapy in breast cancer patients inhibits estrogen activity in the bone as well as breast cancer. Anti-estrogen therapy could induce negative bone balance in breast cancer patients caused by severe estrogen depletion.[7] However, it can significantly increase bone loss compared to physiologic postmenopausal bone loss,[89101112] thus increasing the risk of osteoporotic fractures.[13141516]
Concern about fracture risk induced by anti-estrogen therapy in breast cancer patients is increasing.[1718] However, the fracture risk in patients with breast cancer remains controversial. Data on the fracture risk in breast cancer patients are limited. Thus, the purpose of this study was to determine the risk of osteoporotic fracture in patients with breast cancer through a systematic review and meta-analysis.
METHODS
This study was exempted from review by the institutional review board (IRB) because of its retrospective nature.
1. Search strategy and selection criteria
This meta-analysis was conducted according to the updated guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P).[19] Two researchers (blinded by authors) independently searched Medline (PubMed), EMBASE, and Cochrane Library databases in September 2018. An overview of the search strategy is presented in Supplementary Appendix A. Two authors then independently screened titles and abstracts to identify studies on fractures in patients with breast cancer. They also checked reference lists of all potentially eligible studies and review papers to find additional relevant publications. Articles that met the selection criteria were included in this meta-analysis.
Studies were screened and selected by all investigators based on a priori criteria. Inclusion criteria were as follows: (1) published as an original article in English; (2) evaluated the incidence of osteoporotic fracture (hip, vertebral, distal radius, and proximal humerus) as primary outcomes; and (3) available numerical data for both cases and controls (number of patients, mean and standard deviation of age). Exclusion criteria were as follows: (1) treated for osteoporosis; (2) treated with chemotherapy or hormone replacement; and (3) a review, a case report, or an in vitro study. Two authors (YJI and LYK) reviewed the retrieved full manuscripts to determine whether osteoporotic fracture had been reported in patients with breast cancer. The primary outcome for the meta-analysis was the difference in the incidence of osteoporotic fractures. The osteoporotic fracture included any of hip, vertebral, distal radius, and proximal humerus fractures. The location of the fracture was not distinguished. For every eligible study, the following data were extracted and entered into a spreadsheet by 2 reviewers: family name of the first author, year of publication, country, number of patients, and basic characteristics of subjects (age).
2. Statistical analysis
The primary analysis involved a proportion meta-analysis of the data from all relevant studies that reported the incidence of osteoporotic fracture. A fixed-effects or random-effects model was used to quantify the pooled effect size of included studies, depending on the heterogeneity of the data. Heterogeneity between comparable studies was tested using χ2 and I2 tests. P>0.1 and I2<50%, respectively, were used as established criteria to determine statistical heterogeneity. All analyses were performed using STATA software, version 14.0 (Stata Corporation, College Station, TX, USA).
RESULTS
From PubMed-Medline, EMBASE, and Cochrane Library, a total of 2,161 published articles were found after searching for osteoporotic fracture in patients with breast cancer. Of these 2,161 articles, 1,915 were excluded because of duplication. A total of 204 were then excluded because they did not meet our inclusion criteria (Fig. 1). The remaining 4 studies fulfilling all inclusion criteria were reviewed.[20212223] One was from China, 1 from Taiwan, 1 from the UK, and 1 from Israel. These studies involving 127,722 participants were identified for the meta-analysis.
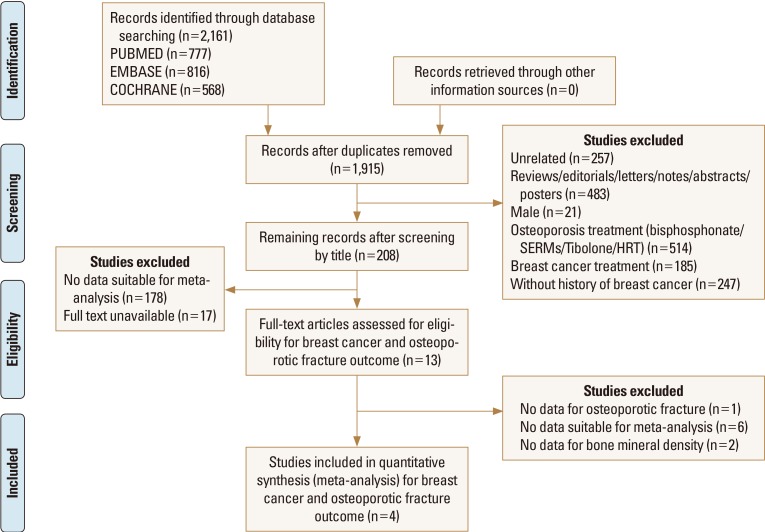
Preferred reporting items for systematic reviews and meta-analysis flow diagram showing the process of selecting relevant studies.
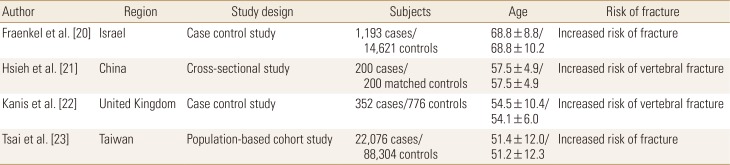
Fraenkel et al. [20] performed a case control cross-sectional, retrospective study to determine whether breast cancer was associated with osteoporotic fracture in 17,110 women with a BMD test between 2003 and 2011. Among 1,193 women with osteoporosis, 62 had a previous history of breast cancer while the remaining 131 did not. BMD was similar among women with and without breast cancer who had fractures. Hsieh et al. [21] performed a cross-sectional, retrospective study to compare the prevalence of vertebral fractures in 200 breast cancer women with age- and body mass index-matched women. They showed 22 (11%) vertebral fractures in breast cancer survivors compared to 7 (3.5%) vertebral fractures in the comparison group. The adjusted odds ratio (OR) for vertebral fracture was 4.16 (95% confidence interval [CI], 1.69–10.21; P<0.01). Kanis et al. [22] have performed a retrospective case-control study to compare the risk of vertebral fractures between women with and without breast cancer. They showed that the incidence of vertebral fracture in women with breast cancer was nearly 5 times greater than that in the control group (OR, 4.7, 95% CI, 2.3–9.9) and 20-fold higher in women with soft-tissue metastases without evidence of skeletal metastases (OR, 22.7, 95% CI, 9.1–57.1). Tsai et al. [23] have performed a nationwide retrospective cohort study to compare the risk of osteoporotic fracture between 22,076 breast cancer patients and 88,304 controls between 2000 and 2003. They showed that the incidence of all types of fractures was higher in the breast cancer cohort than in the comparison cohort, with adjusted HRs of 1.18 (95% CI, 1.03–1.35) for hip fractures, 1.12 (95% CI, 0.98–1.28) for forearm fractures, and 1.24 (95% CI, 1.04–1.48) for vertebral fractures (Table 1).
Analysis for 4 studies involving a total of 127,722 (23,821 cases and 103,901 controls) subjects showed that the incidence of fractures was higher in breast cancer patients than in the control group. The pooled estimate of crude OR for osteoporotic fractures was 1.244 (95% CI, 1.091-1.419; P=0.001; Fig. 2).
DISCUSSION
Clinical implications of menopause in bone metabolism are critical because it results in loss of bone mass.[24] However, the potential adverse effect of breast cancer on bone remains controversial. Our purpose was to review the literature on the fracture risk in breast cancer patients, focusing on the reported occurrence of osteoporotic fracture. After reviewing one population-based cohort study, 1 cross-sectional study, and 2 case-control studies, the fracture risk in breast cancer patients appeared to be higher than that in the general population.
Menopause with estrogen deprivation is the most important cause of osteoporosis in women. Anti-estrogen effects by tamoxifen and aromatase reductase inhibitor (ARI) commonly used to treat breast cancer may lead to menopause even in young women.[25] Although we planned to conduct a structural meta-analysis, the number of included studies was too small. However, we found that breast cancer patients showed a higher risk of osteoporotic fracture through a meta-analysis.
Current reviews have explored the linkage between postmenopausal osteoporosis and breast cancer due to increasing the prevalence of breast cancer and osteoporosis among postmenopausal women.[2627] In fact, in some postmenopausal women, osteoporosis was considered to be a late-term effect of estrogen receptor (ER)-positive breast cancer treatment.[28] A clearer understanding of the linkage between postmenopausal osteoporosis and breast cancer could lead to the development of a therapeutic target for postmenopausal breast cancer patients. Below, we examined some biochemical linkages between postmenopausal osteoporosis and breast cancer.
Receptor activator of nuclear factor-κB ligand (RANKL) is an important cytokine that is a member of the tumor necrosis factor (TNF) family. It is coded for TNF ligand superfamily member 11 (TNFSF11) genes.[29] RANKL plays an important role in human physiology by regulating the differentiation and activation of bone cells called osteoclasts that are responsible for bone breakdown.[26] Osteoclasts can cause a cascade of events culminating in the removal and replacement of low bone content with new bone, preserving bone and skeletal integrity.[30] Reports have shown that when controlled, RANKL can facilitate osteoclast differentiation with subsequent initiation of excessive bone resorption, leading to loss of bone integrity.[26] Ironically, the RANKL/RANK pathway has been implicated in breast development and breast carcinogenesis. In RANK- and RANKL receptor-deficient mice, lactating mammary gland did not develop cancer.[31] In another study, the production of mammary carcinogenesis in 7,12-Dimethylbenzathracene-induced mice was associated with increased RANKL expression.[25] Accelerated breast carcinogenesis was observed in RANK-transgenic mice.[25] These studies clearly point to the involvement of RANKL in mammary tumorigenesis, providing insights into future research that could target RANKL for hormone receptor-positive breast cancer therapy.
Bone and breast tissues are both estrogen-dependent. Previous studies have shown that high bone mineral density (BMD) is correlated with breast cancer risk.[32] Estrogen hormone is a central regulator of bone density. It maintains a balance between bone formation and bone resorption by either reducing osteoclast levels or promoting osteoblast proliferation.[33] Estrogen deficiency has been associated with a decreased BMD that is prominently seen in postmenopausal women.[34] In breast carcinogenesis, increased exposure to estrogen is correlated with early menarche, late menopause, estrogen replacement therapy, and obesity. High blood levels of estrogen can increase the risk, incidence, and severity of breast cancer in premenopausal and postmenopausal women.[35] Molecular studies on possible mechanisms by which estrogen influences breast carcinogenesis have indicated that estrogen acts on the ER, that estrogen-ER dimer binds to the estrogen-responsive component, and that transcription factors such as activator protein-1 are enabled.
In contrast, the specificity protein-1 promotes cell proliferation which ultimately causes cancer cells to spread.[36] In addition, Nishimukai et al. [37] performed study about different patterns of change in bone turnover markers during treatment with bone-modifying agents for breast cancer patients with bone metastases. They reported that tartrate-resistant acid phosphatase 5b (TRACP-5b) appears to affect levels most quickly and sensitively, possibly due to its direct link to the number and activity of osteoclasts. These findings suggest that the efficacy of TRACP-5b is clinically significant when considering which bone-modifying agents to use for breast cancer patients with bone metastases.[37] Due to the role of estrogen in breast cancer, AIs that are inhibitors of aromatase (estrogen metabolizing enzyme) have been used in the treatment of postmenopausal ER-positive breast cancer patients despite challenges to bone fracture in these patients.[32] Recently, scientists are investigating the potential of denosumab, an anti-RANKL antibody, to prevent bone loss associated with AIs. Such studies could provide significant benefits for postmenopausal breast cancer patients.
Although reactive oxygen species (ROS) play important roles in physiological functions, overwhelming levels of ROS generation can result in oxidative stress associated with postmenopausal osteoporosis and carcinogenesis.[38] Osteoclasts recruit ROS to facilitate the catabolism of calcified tissue needed for bone remodeling.[38] Conversely, excess ROS generation results in oxidative stress and causes osteocyte apoptosis which is associated with increased turnover of bone remodeling and bone loss.[3940] It has been shown that hydrogen peroxide can enhance osteoclast activity, further supporting the concept that oxidative stress is associated with increased bone resorption and low bone mass.[41] Studies of antioxidant ability to inhibit bone-associated disorders have shown that glutathione and N-acetylcysteine can reduce osteocyte apoptosis and enhance RANKL expression.[4243] For breast cancer, oxidative stress is involved in the initiation, development, and progression of breast carcinogenesis.[44] Breast tissue is a complex combination of different types of cells, including neoplasm cells and stromal cells.[45] In cancerous breast tissue, stromal fibroblasts develop a phenotype characterized by increased levels of growth factors, cytokines, and metalloproteinases.[46] Altered redox status in favor of pro-oxidants in the tumor microenvironment can induce the development of activated fibroblasts, resulting in changes in epithelial cells which facilitate tumorigenesis.[47] Oxidative stress in the tumor microenvironment is also characterized by activated stromal cells that produce signal-enhancing tumors and promote tumor growth and vascularization.[44] Oxidative stress is therefore clearly involved in both postmenopausal osteoporosis and breast cancer. Serum estrogen levels are decreased by approximately 90% at menopause.[48] In post-menopausal women, anastrozole, letrozole, and exemestane can lower serum levels of estrogen by 81% to 94%, 88% to 98%, and 52% to 72%, respectively.[49] Bone physiology is best understood when described at levels of osteoclasts, osteoblasts and osteocytes (cellular level), bone cell interaction during bone remodeling (tissue level) and bone turnover and mass or fracture incidence. At the cellular level, estrogen has profound effects on osteoblasts and osteoclasts. Estrogen decreases the osteoblastic development of resorptive cytokines including RANKL, colony-stimulating factor-1, interleukin-1, and TNF. At the same time, it increases the production of anti-receptive cytokines (mainly osteoprotegerin), leading to increased osteoclastic apoptosis and increased osteoblastic activity.[50] This results in an increase in osteoclast-stimulating serum parathyroid hormone which leads to increased bone loss.[51] Nonetheless, there is only one study available on the effect of AIs on osteoblast-like cells. It indicates that exemestane can enhance osteoblast behavior.[52] Considering the mechanism, additional meta-analysis related to ARI or tamoxifen is necessary.
CONCLUSIONS
Overall, our results suggested that patients with breast cancer might have an increased risk of osteoporotic fractures, although the number of studies included for the meta-analysis was small. Larger-scale better-designed studies that report the occurrence of osteoporotic fracture in breast cancer patients are needed in the future to determine which factors are associated with an increased risk of osteoporotic fracture in patients with breast cancer.
Acknowledgments
This research was supported by a grant (HI18C0284) from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea.
Notes
Ethics approval and consent to participate: Not applicable.
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
SUPPLEMENTARY MATERIAL
Supplementary Appendix 1
Detailed search strategies for each database. MeSH terms, search terms, and combinations of the 2 were used for each database search