Sarcopenia: Neurological Point of View
Article information
Abstract
Sarcopenia is an age-related geriatric syndrome which is characterized by the gradual loss of muscle mass, muscle strength, and muscle quality. There are a lot of neurologic insults on sarcopenia at various levels from the brain to the neuromuscular junctions (NMJs) to generate a volitional task. Dopaminergic downregulation, inadequate motor programming and motor coordination impairment lead to decline of supraspinal drive. Motor unit reorganization and inflammatory changes in motor neuron decrease conduction velocity and amplitude of compound muscle action potential. Furthermore, NMJ remodeling and age related neurophysiological alterations may contribute to neuromuscular impairment. Sarcopenia is an age-associated, lifelong process which links to multiple etiological factors. Although not all the causes are completely understood, we suggest that compromised nervous system function may be one of the important contributors to the sarcopenia.
INTRODUCTION
Sarcopenia is an age-related geriatric syndrome which is associated with subsequent disability and morbidity. Sarcopenia which is coded recently in the International Classification of Disease, Tenth Revision (ICD-10) Clinical Modification (CM), is characterized by the gradual loss of muscle mass, muscle strength, and muscle quality in aging.[1]
In understanding the mechanisms of loss of mobility and physical performance, some studies have focused on muscle mass represented by lean body mass.[234] However, a number of these studies that try to test the hypothesis that decline in muscle mass was the main cause of mobility and physical function decline in late life showed surprising results. Muscle mass was not a good predictor of physical function. It was highly related with muscle strength, but not as much as expected. Furthermore, the decline of muscle strength was more severe than that of muscle mass in aging.[5678] These studies suggested that not only decline in muscle mass, but also decline in muscle quality would cause the decrease in muscle strength. Even though strength is most direct measure of the contribution of muscle movement, it can explain only a small part of decline of physical performance with aging. In this context, it is a matter of course that there are no effective treatment for sarcopenia which include pharmacological interventions attempt to increase muscle mass only.[91011] Therefore, it is important to explore deeper into the mechanism or etiology of sarcopenia, other than muscle itself, which in turn will provide further insight into the development of more effective therapy.[12]
Given that motor afferent supply is essential for muscle contraction, and function, surprisingly little is known about how this neurogenic component affects or is influenced by muscle aging. During a voluntary movement, adequate control of muscle contraction in different muscle groups is a very complex process, which requires the coordination with other systems. Now, we have some attention to the neurological control of the movement as an important cause of sarcopenia.
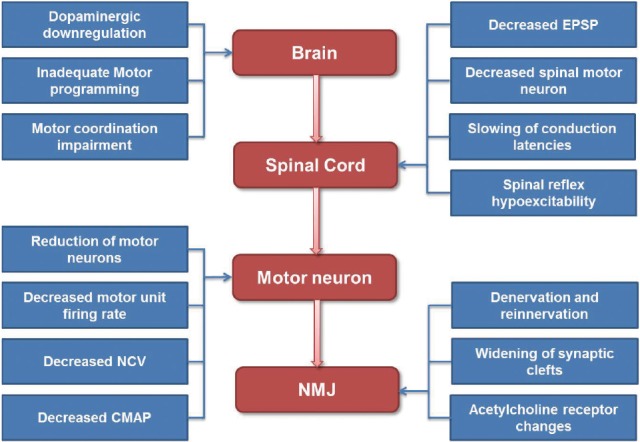
There are a lot of neurologic insults at various levels from the brain to the neuromuscular junctions (NMJs) to generate a volitional task (Fig. 1). In this article, we will review the neurological component of sarcopenia by following the chain of events that leads to the generation of a voluntary movement in normal aging process (Fig. 2).
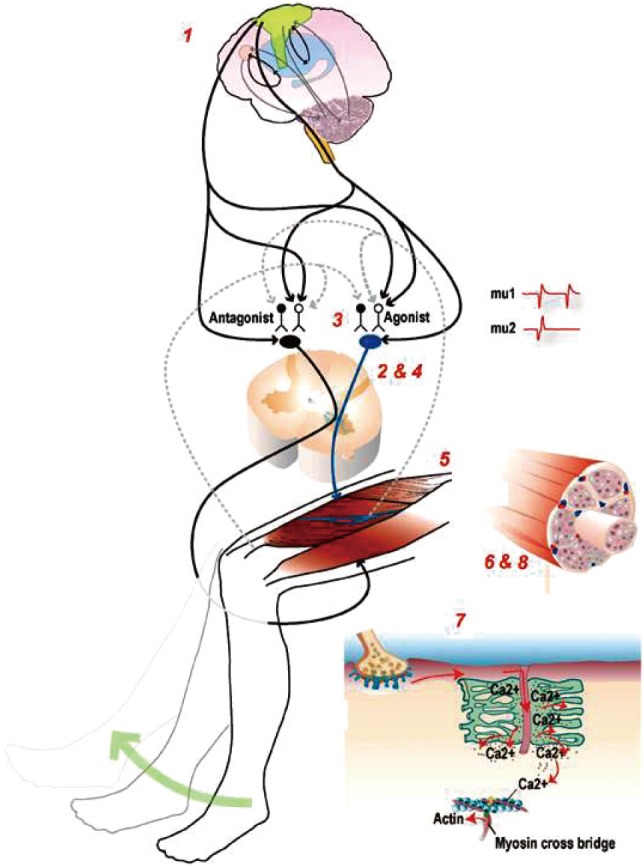
Potential sites and physiological mechanisms that regulate strength. The neuromuscular system contains several potential sites that can affect voluntary force or power production, such as 1) excitatory drive from supraspinal centers, 2) α-motoneuron excitability, 3) antagonistic muscle activity, 4) motor unit recruitment and rate coding, 5) neuromuscular transmission, 6) muscle mass, 7) excitation-contraction coupling processes, and 8) muscle morphology and architecture. [Reprinted from “Sarcopenia =/= dynapenia”, by Clark BC and Manini TM, 2008, J Gerontol A Biol Sci Med Sci, 63, pp.829-34.
CENTRAL NERVOUS SYSTEM
1. Motivational systems
Previous studies have demonstrated age-related changes in the dopaminergic system. Aging lead to decrease level of dopamine, reduce the postsynaptic dopamine receptors and presynaptic dopamine transporters which collectively result in altered reward processing even in healthy human subjects.[131415] The down-regulated dopamine signal with aging results in diminished reward signals and leads to decrease motivation for movement.[16] Reduced motivation would bring on reduced physical activity.
2. Motor programming and executive function
In older adults, motor control relies on more widespread engagement of the prefrontal cortex and basal ganglia networks. Unfortunately, these systems are the most detrimentally affected by the aging process.[16] The prefrontal cortex and basal ganglia make a loop that plays a predominant role in motor planning and execution.[17] This loop is directly involved in the motor programming of movement, such as during movement preparation and execution or when switching between complex coordinated movements, and the prefrontal regions dealing more directly with the direction and the extent of movement.[18,19] Voxel-based morphometry analysis showed age-related atrophy in gray and white matter volume most prominently in frontal cortex and this structural change would lead to frontal dysfunction which is associated with motor dysfunction.[20] Additionally, age-related dopaminergic degeneration, which has been shown to decrease performance on executive function, may indirectly contribute to age related motor dysfunction and underlie decreased fine movement control and movement slowing.[212223]
3. Motor coordination
Subcortical structures such as basal ganglia and cerebellum are important for motor performance and coordination. Supplementary motor area and cingulate cortex also play a role in preparing adequate movement. These regions exhibit accelerated decrease in volume with age.[24]
4. Spinal cord
Little is known about changes in spinal cord and spinal cord neurons with aging. The volitional motor program made in various system leave the brain from primary motor cortex to spinal motoneurons through the corticospinal tracks. Although many other measures of corticospinal communication appear unaffected by aging, the excitatory postsynaptic potentials (EPSP) in spinal motoneurons which is induced by fast-conducting descending volleys show a linear decline with age.[25,26] Animal studies found signs of spinal cord degeneration, including demyelination of individual axons starting in the ventral roots and later developing in the dorsal roots followed by fibrosis. Autopsy studies in humans have shown that the number and diameter of anterior horn motor neurons decline with increasing age. [272829] The estimated number of spinal motor neurons was declined about 25% to 50% between the age of 20 and 90, with most of the loss coming in later years. The loss of motor neurons is associated with an increase in the number of astrocytes and with an apparent alteration in the dendritic networks of the neurons.[30] The conduction latencies in the spinal cord are slowing and transcranial magnetic stimulation studies have shown reduced excitability of efferent corticospinal pathways in elderly.[31] These changes may cause reduction of muscle strength, mass and performance with aging.
PERIPHERAL NERVOUS SYSTEM
1. Motor unit
One of mechanisms the nervous system can regulate strength is behavioral modulation of the motor unit pool such as recruitment, discharge rate and synchronization of motor units.[2] The primary way to control exerted muscle force is via recruitment of additional motor units within a given α-motoneuron pool and/or increased motor unit discharge rate. With aging there are many changes in the neuromuscular system, which may impact the occurrence of doublet discharges. At first, as the number of motor units is decreased their size is increased due to increased number of fibers per motor unit, which in turn increases the force generated by that unit.[32,33] Thereby, motor unit size and firing rate are already higher at low force levels with a progressive recruitment of larger units as force increases. In spite of this compensatory reorganization, the ability to produce intended force declines with age. At last, there is a decrease excitability of motor neurons, and decreased maximal firing rate of motor units with aging.[34,35] A study assessing the force and discharge characteristics of motor units demonstrated that the recruitment of successive motor units does not differ between age groups, but that motor unit recruitment may be more transient in older persons and contribute to the greater variability in force observed in old adults during graded ramp contractions.[36] Another study shows that age is associated with an increase in the size of the motor unit and a decline in motor unit firing rate during sustained quadriceps muscle activation at effort levels relevant to daily mobility tasks. However, these changes were particularly notable in the oldest subjects who used larger units per unit force and higher firing rates per unit force than younger subjects at low force levels. These observations would suggest that the altered motor unit activation appears later in life than the age at which sarcopenia develops.[33]
2. Motor neuron
The nerve conduction velocities (NCVs) of peripheral nerves are decreased after 30 to 40 years of age. There is a gradual decline of the amplitude of compound muscle action potentials (CMAPs) and sensory nerve action potentials.[37] The reduction in conduction velocity is due to drop outs of the largest axonal fibers and reduced myelination in part. These changes in the conduction velocities with age are related to loss of muscle strength. Previous study about the relation of age-related changes in the median nerve function to grip strength found that median NCV declined linearly with age and has an independent contribution to age-associated levels of muscle strength. The level of the effect was smaller than what could be attributed to forearm muscle mass or age.[38] Cross-sectional study examined the relationship of peroneal NCV and CMAP with calf muscle mass and density, two complementary measures of sarcopenia showed that both nerve and muscle parameters declined with age although in most cases the decline was not linear. In both sexes, CMAP was independently and significantly associated with calf muscle density and these findings suggest that intrinsic changes in the muscle tissue are partially caused by a reduction in the number of motor axons.[39]
Insulin-like growth factor I (IGF-1) has potent effects on motor axon myelination, motor neuron apoptosis, stimulation of axonal sprouting, and repair of damaged axons.[40] Elevated levels of inflammatory cytokines tumor necrosis factor-α (TNF-α) and TNF-β, which is typically observed in old individuals, can blunt the IGF-1-mediated effects in muscle tissue and might have similar effects in motor neurons and its axons.[41]
3. NMJ
Age-related changes also occur at the level of the NMJ. Some, but not all, denervated muscle fibers are reinnervated through collateral sprouting of nearby surviving motor axons or motor end plates, which results in the formation of very large motor units.[42] As a response to this partial denervation of myofibers within a motor unit, the intact axons sprout to reinnervate surrounding denervated muscle end plates. Perisynaptic Schwann cells at the NMJs) extend processes that bridge between denervated and reinnervated endplates, and guide axonal sprouts to reinnervate the denervated endplates.[43] IGF-1 also facilitates collateral sprouting of surviving axons to innervate denervated muscle fibers, and reinnervation of muscle fibers via axonal sprouting.[44] However, animal studies have suggested that the capacity for axonal and motor endplate sprouting is relatively limited with aging.[45] Neuromuscular inactivity also seems to produce inhibition of axonal sprouting in partially denervated muscle, likely due to failure of perisynaptic Schwann cell processes to bridge and guide axonal sprouts to reinnervate denervated endplates, and/or caused by a reduced calcium influx into nerve terminals to support sprout growth.[43] Interestingly, with very high daily neuromuscular activity, the large levels of acetylcholine released from intact endplate terminals could suppress glial fibrillary acidic protein synthesis in perisynaptic Schwann cells leading to impaired axonal growth between innervated and denervated terminals.[46] Thus, normal aging appears to be characterized by a temporal progression of motor unit loss accompanied by adaptive axonal sprouting, which is gradually replaced by maladaptive peripheral sprouting. Furthermore, the endocrine and paracrine production of IGF-1 which stimulates axonal sprouting is reduced in elderly individuals.[40] In turn, the gradual impairment in axonal sprouting capacity at old age results in a progressive loss of motor endplate terminals, which initiates an accelerated loss of muscle fibers.[42]
Further, age-related remodeling of motor units appears to involve denervation of type II (fast) skeletal muscle fibers with collateral reinnervation allowing for the type I (slow) motor units to gain control of the denervated muscle fibers (Fig. 3). As the fast-twitch motor units are the determinant of the degree of power exerted by muscles, their loss in aging contributes to the loss in muscle power.[47] If denervation outpaces reinnervation, a population of denervated fibers will undergo atrophy and degeneration due to the loss of trophic factors.[48,49] This process contributes to the loss of muscle mass at least partly by apoptosis.[1,50]
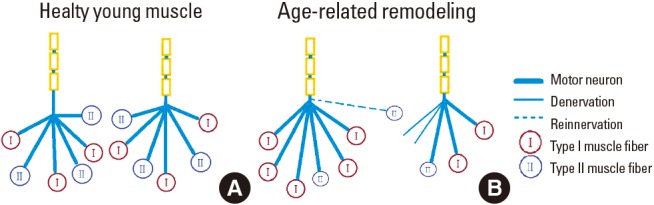
Effect of age on the motor unit, depicting, young and aged motor units. This drawing depicts the pronounced denervation of type II fibers and the recruitment of type I fibers into surviving motor units in older subjects.
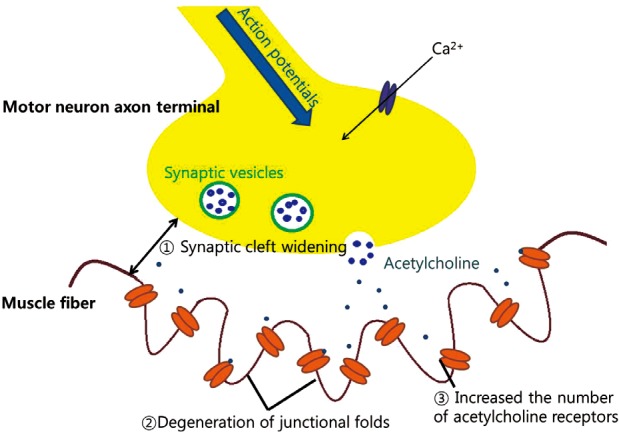
Anatomical remodeling at NMJ is also occurred in older persons (Fig. 4). Widening of synaptic clefts and degeneration of junctional folds at the post synaptic region had been reported.[51] Furthermore, the distribution and morphology of acetylcholine receptors changes with age. The number of perijunctional acetylcholine receptors is increased and a greater number of smaller complex of acetylcholine receptors are seen in older persons.[52] These anatomical remodeling changes may lead a more rapid rundown of endplate potential strength during continuous stimulation of the pre-synaptic neuron.
CONCLUSION
Sarcopenia is an age-associated, lifelong process which links to multiple etiological factors. Although not all the causes are completely understood, we suggest that compromised nervous system function may be one of the important contributors to the sarcopenia. From the generation of neural signal to making a muscle contraction, there are a lot of age-associated changes in nervous system which may affect motor performance, muscle strength and muscle mass. How to slow down this neural aging process is critical problem and the answer can be one of the treatment options of the sarcopenia.
Notes
No potential conflict of interest relevant to this article was reported.