Olecranon Fractures Have Features of Osteoporotic Fracture
Article information
Abstract
Background
To determine whether olecranon fractures have osteoporotic features such as age-dependent, low bone attenuation and low-energy trauma as a cause of injury.
Methods
Elbow computed tomography (CT) and medical record review were performed in 114 patients (53 males and 61 females) with acute olecranon fractures. The mean age was 57 years. Bone attenuation was measured on the central part of the olecranon on sagittal CT images avoiding the fracture, and on the distal humerus (distal metaphysis and medial and lateral condyles) on coronal CT images. We compared bone attenuation and causes of injury (high or low energy trauma) between younger (<50 years) and older (≥50 years) patients in each gender. Multiple regression analysis was performed to determine the effect of age and gender on bone attenuation.
Results
Mean bone attenuation in older male and female patients was significantly lower than in younger patients, except at the medial condyle in men. The proportion of low-energy trauma in older male patients was significantly higher than in younger male patients. In female patients, low-energy trauma was predominant in both younger and older patients. Age and female gender had significantly negative effects on bone attenuation.
Conclusions
This study demonstrated that olecranon fractures have osteoporotic features, including age-dependent low bone attenuation and low-energy trauma as the predominant cause of injury. Our results suggest that osteoporosis evaluation should be considered for patients aged 50 years or more with olecranon fractures.
INTRODUCTION
Osteoporosis, a serious systemic disease, is associated with increased morbidity, mortality, and economic cost.[1234] Osteoporotic (fragility) fractures are fractures resulting from low-energy mechanical forces that would not normally result in a fracture according to National Institute for Health and Care Excellence clinical guidelines.[5] Several studies have suggested that the vertebrae, hip, proximal humerus, and distal radius fractures are major osteoporotic fractures.[46] Fractures of the femur diaphysis, ankle, and rib have also been included as osteoporotic fractures.[78910] Previous studies have shown that history of an osteoporotic fracture is a risk factor of future fracture.[11112131415] Therefore, patients with a prior fracture should receive further evaluation for osteoporosis and fracture risks.
Although fractures around the elbow are common,[16] there is less evidence showing that they have features of osteoporotic fracture. A few studies have mentioned that radial head and distal humerus fractures are potentially osteoporotic fractures. [17181920] However, whether olecranon fractures have osteoporotic features have not been evaluated yet. Therefore, the objective of this study was to determine whether olecranon fractures might have features of osteoporotic fracture such as age-dependent low bone attenuation and low-energy trauma as the cause of injury.
METHODS
1. Subjects
This study was approved by the Institutional Review Board of our hospital. We reviewed consecutive patients with acute olecranon fracture between March 2004 and February 2016 who were treated at our institution, an urban tertiary referral hospital. The inclusion criteria were: 1) acute olecranon fracture; 2) age of 20 years or more; and 3) available computed tomography (CT) scan of the elbow and medical record that documented the causes of injury. Exclusion criteria were: 1) avulsion fracture; 2) interval of more than 1 week between injury and CT examination; 3) CT scan taken at other hospitals; 4) rheumatoid arthritis, previous elbow trauma history, or any other conditions that could affect bone quality around the elbow.
A total of 114 patients who met the inclusion criteria were analyzed, including 53 males and 61 females. Their mean age was 57 years with standard deviation (SD) of 20 years. The causes of injury were categorized into high-energy trauma and low-energy trauma. Motorcycle accidents, car traffic accidents, fall from a height over a standing height, and sports accidents such as skiing were considered as high-energy trauma whereas slips and falls from a height less than a standing height were considered as low-energy trauma.[21]
2. CT measurement
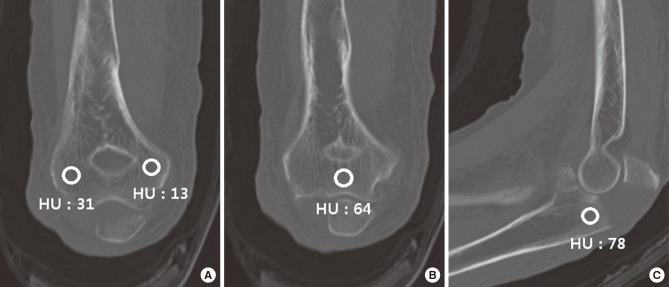
Elbow CT examinations were performed using Brilliance iCT (Philips Healthcare, Cleveland, OH, USA) at 120 or 140 kVP with patient in supine position. CT images were obtained and measurements were performed using picture archiving and communication system (PACS). Bone attenuation was measured by placing a circular region of interest (ROI) on sagittal images of the olecranon and at the distal humerus (distal metaphysis and medial and lateral condyles) on coronal images where each region appeared to be the largest (Fig. 1). A circular ROI with a diameter of 3 mm was manually placed on the cancellous portion of each region while avoiding subchondral bone area and fracture line. Mean bone attenuation of each region was recorded as Hounsfield units (HU). Bone attenuations were measured by one of the authors who was blinded to the patient's information. For inter- and intra- observer reliability, CT images of 20 patients were selected randomly. For these 20 patients, the above-mentioned author measured bone attenuation once again after an interval of 4 weeks for intra-observer reliability. Another author measured the bone attenuation to determine inter-observer reliability.
3. Analyses
Patients were divided into two age groups. The older age group consisted of patients who were 50 years of age or older. The younger age group consisted of those who were less than 50 years old. We chose this age as a cut-off value because several previous studies have used the age of 50 as the cut-off value for osteoporosis evaluation. In addition, it has been reported that bone mineral density (BMD) remains fairly constant until about 50 years of age.[722232425] Bone attenuation between groups was compared using Student's t-test, after confirming the normality of the data by variance-equivalence test. The proportions of high- or low- energy trauma were compared between groups with χ2 test.
The effects of age and gender on bone attenuation of the olecranon and the distal humerus were analyzed for all patients by multiple linear regression analysis. Independent variables were age and gender. Dependent variables were bone attenuations of the olecranon, lateral condyle, medial condyle, or the center of the distal humerus. In addition, the relationships between bone attenuation of the olecranon and age in both genders were assessed by spearman's correlation coefficient.
To determine the statistical power, primary outcome variable was bone attenuation on the olecranon. To determine a difference of 87 HU (50% of the mean bone attenuation) between two groups with a pooled SD of 82 HU (for an effect size of 1), a power analysis indicated that a sample size of 18 patients in each group would provide 80% statistical power to detect this effect size between groups (α=0.05, β=0.2) using t-test. Thus, the number of patients in each group of this study satisfied the statistical power. All statistical analyses were performed using the SPSS version 23.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was considered when P-value was less than 0.05.
RESULTS
1. CT measurement
Mean bone attenuations of the olecranon, the lateral condyle of the distal humerus, the medial condyle, and the center were 174 (SD, 82), 130 (SD, 90), 117 (SD, 95), and 140 (SD, 69) HU, respectively. In male patients, the mean bone attenuation of the older age group was significantly lower than that of the younger age group in the olecranon (P=0.014), lateral condyle (P=0.013), and the center of the distal humerus (P=0.011) (Table 1). The difference in the medial condyle of the distal humerus was not statistically significant (P=0.056) between the two age groups. In female patients, the mean bone attenuations of the older age group were significantly lower than those of the younger age group in all measured areas (P<0.001).
2. Cause of injury
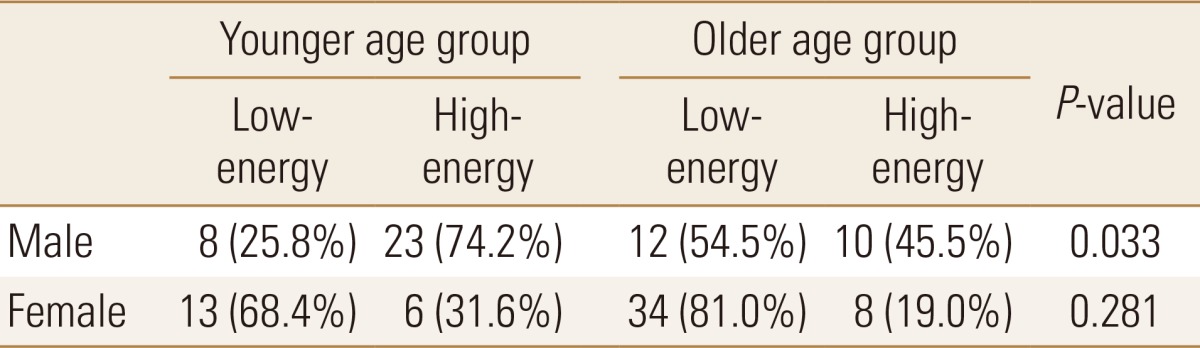
In male patients, the proportion of olecranon fractures caused by low-energy trauma in the older age group was significantly higher than that in the younger age group (P=0.033) (Table 2). In female patients, low-energy trauma was more common in both younger and older patients, with no significant intergroup difference (P=0.281).
3. Effect of age and gender on bone attenuation
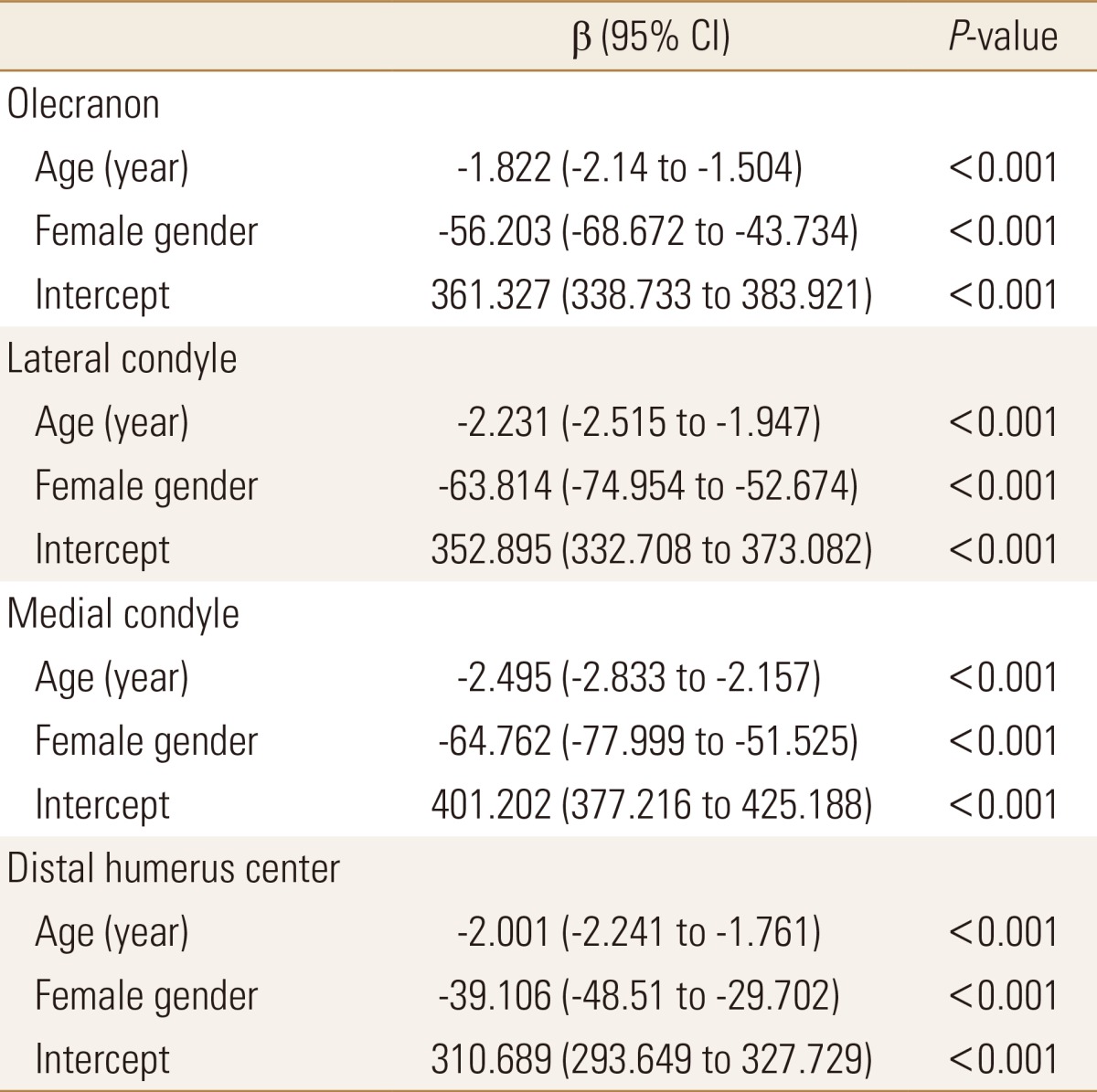
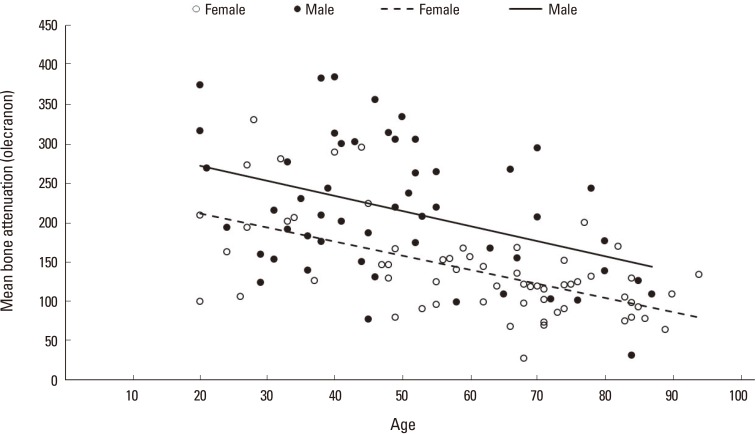
Multiple linear regression analysis revealed that age and female gender independently had negative effect on bone attenuation (Table 3). In spearman's correlation coefficient, mean bone attenuation of the olecranon was negatively correlated with age in both male (r=-1.909, P=0.003) and female (r=-1.772, P<0.001) patients (Fig. 2).
4. Reliability of CT measurement
Intraclass correlation coefficient (ICC) values for intra- and inter- observer reliability are shown in Table 4. All ICC values were >0.8, indicating excellent inter- and intra- observer reliabilities.
DISCUSSION
Several studies have suggested that fractures of the vertebrae, hip, proximal humerus, and distal radius are major osteoporotic fractures. Fractures of the femur diaphysis, ankle, and rib also have features of osteoporotic fracture.[7891017181920] However, whether olecranon fractures have osteoporotic features has not been reported yet. This study demonstrates that olecranon fractures also have osteoporotic features such as age-dependent low bone attenuation and low-energy trauma as a cause of injury.
We used elbow CT as a surrogate examination of BMD at the olecranon and the distal humerus. Several studies have demonstrated that the average bone attenuation on CT of the spine is correlated with BMD on dual energy X-ray absorptiometry (DXA).[262728] In addition, it has been reported that bone attenuation on CT of the ankle or the wrist can be used as marker of bone quality.[72930] Romme et al.[26] have found that bone attenuation of the thoracic spine on routine chest CT is correlated strongly with BMD in patients with chronic obstructive pulmonary disease. They have reported that the attenuation threshold of 147 HU has the highest sensitivity and specificity for osteoporosis.[26] Schreiber et al.[27] have also evaluated CT of the distal radius and found that the attenuation threshold at 218 HU for females has optimum sensitivity and specificity for increased risk of distal radius fracture. Our results revealed that the mean bone attenuation of the elbow in women in the older age group ranged from 65 HU (SD, 43 HU) in the lateral condyle to 114 HU (SD, 35) in the olecranon, which were relatively low compared to the reported attenuation threshold HU values for osteoporosis in the thoracic spine or the wrist. Further studies correlating bone attenuation on elbow CT with BMD on DXA are needed to confirm their relationship and determine the HU threshold for detecting osteoporosis.
This study has several limitations. First, the number of patients was not large. In addition, patients included in this study were all Koreans recruited from a tertiary referral hospital. Therefore, the population in this study may not represent the general characteristic of all patients with olecranon fracture. However, evaluation with a single CT scanner made the measurement of bone attenuation reliable in this study. Second, this study did not involve control group. Elbow CT examinations could not be performed for age- and sex-matched patients without olecranon fracture because of radiation exposure. In addition, patients who had elbow CT scan without fracture had a certain condition that could affect bone quality such as rheumatoid arthritis. Third, analysis of bone attenuation involved only cancellous bone. However, both cancellous and cortical bones are important for mechanical strength. High-resolution peripheral quantitative CT that can quantify cortical porosity in vivo may further reveal cortical bone quality of the olecranon.[31] Fourth, olecranon fractures typically occur from a direct blow to the proximal ulna or indirectly from forceful contraction of the triceps against resistance during a fall. However, some fractures can occur when the elbow is hyperextended as the olecranon is impinged at the olecranon fossa of the distal humerus.[32] The exact mechanism of injury could not be confirmed in this study based on medical record. The injuries that involve contraction of the triceps or hyperextension of the elbow might not be classified into low or high-energy trauma based on the height of a fall. Further studies on injury mechanism and energy level needed to cause fracture are needed to determine whether such injuries are also osteoporotic fractures.
In conclusion, our results demonstrate that olecranon fractures have features of osteoporotic fracture, including age-dependent low bone attenuation in both genders and low-energy trauma as predominant cause of injury in men aged 50 years or more and in women regardless of younger or older. Our results suggest that patients aged 50 years or more with olecranon fracture should be evaluated for osteoporosis and fracture risks for secondary prevention of subsequent fractures.
ACKNOWLEDGEMENTS
This study was in part supported by a research fund (2015-R1D1A1A01058562) from National Research Foundation of Korea.
Notes
No potential conflict of interest relevant to this article was reported.