Gestational Diabetes Mellitus, Fetal Growth and Vitamin D
Article information
Abstract
Vitamin D is an important secosteroid hormone in skeletal and non-skeletal systems. Vitamin D has relevance to muscle and immune function, hypertension, diabetes mellitus, cancer, and pregnancy because vitamin D receptors (VDR) are present in many non-skeletal tissues. Vitamin D acts on target tissues via the binding of its active form to VDR. As vitamin D affects not only bone metabolism but also glucose metabolism, vitamin D deficiency may affect the development of gestational diabetes mellitus and fetal growth. Although vitamin D deficiency is prevalent during pregnancy, there are conflicting reports on the effect of vitamin D deficiency on pregnancy complications, such as fetal growth restriction and gestational diabetes. This article reviews published papers on the effects of vitamin D on gestational diabetes and fetal growth.
INTRODUCTION
In non-pregnant state, once vitamin D is synthesized from the skin or diet, it binds to vitamin D binding protein (DBP) and is transferred to the liver. Conversion of vitamin D to 25-hydroxy-vitamin D (25[OH]D) through 25-hydroxylation is taken place in the liver. Since the half-life of 25(OH)D is 2 to 3 weeks, serum 25(OH)D reflects the vitamin status of the body. And 25(OH)D is bounded to DBP and enters the circulation to the kidney where take place 1-hydroxylation of 25(OH)D to 1,25 dihydroxy-vitamin D (1,25[OH]2D) by the action of 1α-hydroxylase, and the half-life of 1,25(OH)2D is 8 hr.
In pregnant state, 25(OH)D crosses the placenta and 1,25(OH)2D passes only at low concentrations during pregnancy. However, 1,25(OH)2D of production is increased primarily through the elevation of 1-hydroxylase activity in the placenta and decidua, which is supplied to mother and fetus.[1] Although adequate vitamin D status is important during pregnancy, it is still controversial which level of serum 25(OH)D during pregnancy is optimal. Because the optimal level of serum 25(OH)D during pregnancy was based on the general population,[23] the definition of vitamin D deficiency in pregnancy is also to be elucidated. Inadequate maternal vitamin D status has known to be associated with several adverse maternal-fetal outcomes, including pre-eclampsia, gestational diabetes mellitus (GDM), increased rate of primary cesarean section, preterm labor, fetal growth retardation, and neonatal hypocalcemia.[4] However, the effects of vitamin D on maternal-fetal outcomes are different based on several clinical trials. The association between vitamin D and GDM has been investigated in several studies which results were still inconclusive due to factors, such as selection bias, timing and methodology of vitamin D measurement, time of GDM diagnosis and diagnostic criteria for GDM.[5] Since maternal 25(OH)D crosses the placenta during pregnancy, it is assumed maternal vitamin D status could affect fetal bone growth, however, the effect of vitamin D on fetal bone growth during pregnancy is sparsely investigated.
VITAMIN D AND GDM
GDM is diabetes that is first diagnosed in the second or third trimester of pregnancy that is not overt diabetes mellitus (DM) prior to pregnancy. If women are diagnosed with DM in the first trimester, their DM should be stratified as having preexisting type 1 or type 2 diabetes rather than GDM.
As the prevalence of GDM which ranges from 2% to 20% depending on the populations is increasing worldwide [5] and risk for developing DM in the postpartum 10 to 20 years is substantial (35–60%), the interest in GDM is growing rapidly. There are several evidences supporting a role for vitamin D in developing glucose intolerance and type 2 DM. Potential mechanisms of effects of vitamin D on glucose metabolism are as follows; the binding of active form of vitamin D to vitamin D receptors (VDR) on pancreatic beta-cells, the expression of 1-α-hydroxylase in pancreatic β-cells, insulin secretion and sensitivity by regulating extracellular calcium and calcium flux through the pancreatic β-cell, the presence of vitamin response element in the human insulin gene promoter, the effects on stimulating the expression of insulin receptor and the effects on systemic inflammation by modulating the effects of cytokines for beta cell function,[6] since insulin resistance (IR) and β-cell apoptosis could be induced by systemic inflammation.
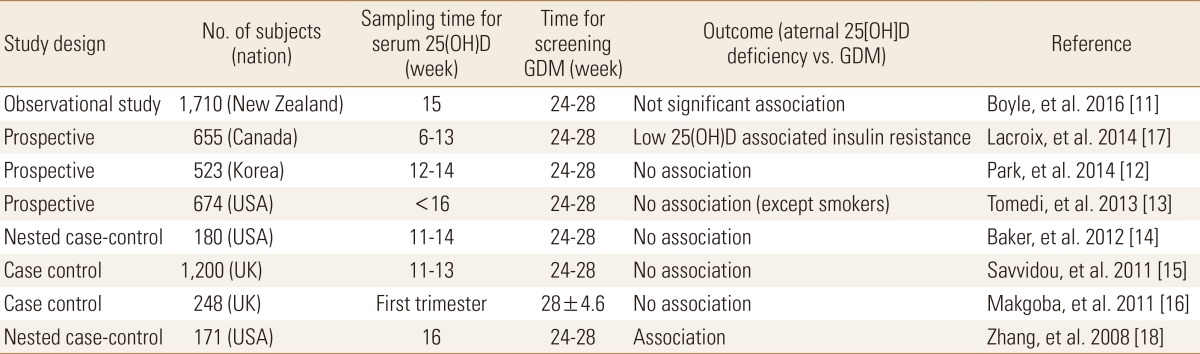
The relevant explanation for molecular mechanisms of vitamin D on type 2 DM [78] and high prevalence of vitamin D deficiency during early pregnancy [9] applying the definition of vitamin D deficiency (serum 25[OH]D level <20 ng/mL) set forth by Institute of Medicine (IOM) suggest that vitamin D deficiency may associate with the development of glucose intolerance or GDM. Therefore, many studies have been focused on maternal vitamin D level and GDM because GDM is associated with adverse pregnancy outcome such as macrosomia, preeclampsia and cesarean section.[710] GDM is diagnosed at the second or third trimester of pregnancy and is therefore likely to be associated with the 25(OH)D status of early pregnancy. Several studies observed the associations between maternal serum 25(OH)D in the first trimester and developing GDM after second trimester were as shown in the Table 1. Boyle et al.[11] showed maternal 25(OH)D levels <30 ng/mL at 15 weeks of gestation were associated with the development of GDM, but after adjustment with body mass index (BMI) and ethnicity, the association was not significant. Park et al.[12] measured maternal 25(OH)D, plasma insulin and glucose at 12 to 14 weeks of gestation followed by calculating homeostatic model assessment (HOMA)-IR and HOMA-B which represented IR and β-cell function among 523 Korean pregnant women including GDM and non-GDM. They showed maternal 25(OH)D were not associated with the risk of GDM, IR and β-cell impairment. Tomedi et al.[13] found an association between maternal 25(OH)D and hyperglycemia only among smokers. Several studies reported maternal vitamin D deficiency was not associated with development of GDM.[141516] In contrast, other studies reported the first trimester maternal vitamin D deficiency was associated with risk for GDM development.[1718] Although the inverse association between vitamin D status and GDM was accepted in previous studies of meta-analysis,[1920] those results are still conflicting because of confounding factors such as ethnicity, definition of vitamin D deficiency, sampling time for serum 25(OH)D (1st, 2nd or 3rd trimester) and study types. A recent report on summary of several meta-analyses have not shown a clear answer to the relationship between vitamin D and GDM.[21]
Because the results of association between maternal vitamin D status during early pregnancy and GDM are still inconsistent, more data are needed to clarify the relationship between maternal vitamin D status and GDM.
VITAMIN D AND FETAL GROWTH
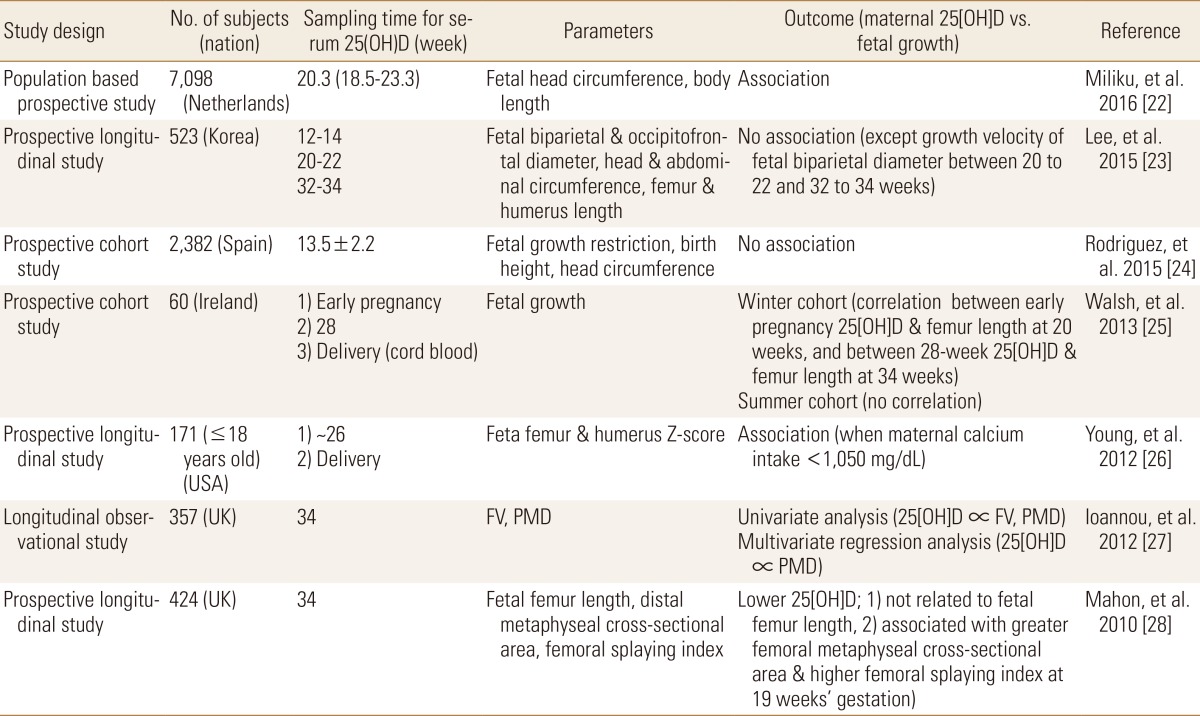
Vitamin D is known to be essential for the health of pregnant women and their fetuses. Low maternal 25(OH)D status decreased transfer of 25(OH)D to the fetus, and may result in impaired growth, delayed bone ossification and congenital rickets.[2] Vitamin D deficiency (serum 25[OH]D <20 ng/mL) among pregnant women is common worldwide, however, optimal level of serum 25(OH)D for fetal growth during pregnancy is still unknown. Although suboptimal maternal vitamin D has been known to be associated with adverse pregnancy outcomes including fetal growth restriction, reports regarding the association between maternal vitamin D and fetal bone growth are inconclusive. In several studies, maternal 25(OH)D has been associated with fetal growth, but other studies reported different results (Table 2). Miliku et al.[22] reported that a low 25(OH)D level in mid-trimester pregnancy is associated with a smaller head circumference and shorter body length during third trimester pregnancy, which reflect a fetal growth restriction. Lee et al.[23] found only a correlation between the difference of serum 25(OH)D level between 12 to 14 and 20 to 22 weeks and the growth velocity of the fetal biparietal diameter between 20 to 22 and 32 to 34 weeks of gestation. Other parameters for fetal growth were not associated with maternal serum 25(OH)D which were measured at first, second and third trimester. Fetal growth such as fetal biparietal and occipitofrontal diameter, head and abdominal circumference, femur and humerus length using ultrasonography were measured three times during pregnancy to generate growth velocity.[23] Rodriguez et al.[24] compared maternal 25(OH)D levels with fetal growth, birth weight, length, and head circumference, and found that high serum maternal 25(OH)D levels were not statistically significant but tended to have a low head circumference, and not significantly associated between 25(OH)D levels and other birth outcomes. Walsh et al.[25] found a correlation between maternal 25(OH)D in early pregnancy and femur length at 20 weeks of pregnancy, and between maternal 25(OH)D level at 28 weeks and femur length at 34 weeks of pregnancy in winter cohort not in summer cohort. And a shorter infant length was found in those with lower serum 25(OH)D levels (less than the median values) at early pregnancy. Young et al.[26] showed fetal femur and humerus z-score in pregnant women under 18 years old were significantly associated with maternal 25(OH)D in case of maternal calcium intake is less than 1,050 mg/day. Ioannou et al.[27] investigated the relationship between fetal femur volume and maternal vitamin D level, fetal femur volume was measured using femur length, proximal metaphyseal diameter, and midshaft diameter. At 34 weeks of gestation, maternal serum 25(OH)D correlated significantly with fetal femur volume and proximal metaphyseal diameter, however, in multiple regression analysis, maternal serum 25(OH)D was statistically significant only with the proximal metaphyseal diameter. In Mahon et al.'s study[28], maternal 25(OH)D measured at 34 weeks of gestation was compared with fetal femur length and distal metaphyseal cross-sectional area using a high-resolution 3D ultrasound and femoral splaying index reflecting fetal femoral morphology. There was no correlation between low maternal 25(OH)D levels and fetal femur length, but it was associated with higher femoral splaying index (which is commonly observed in childhood rickets) at 19 weeks of gestation. It suggested that vitamin D could affect fetal femoral morphology development from the 19th week of gestation.[28] In a meta-analysis studies, Galthen-Sorensen et al.[29] suggested that low maternal 25(OH)D may influence fetal bone growth when maternal calcium intake is low and Pérez-López et al.[30] reported that vitamin D supplementation during pregnancy may increase maternal 25(OH)D levels, birth weight, length, but it was not associated with other maternal and fetal outcomes. As aforementioned, it is very hard to answer whether maternal 25(OH)D and fetal bone growth are associated or not, because several factors such as differences in study design, ethnicity, country, 25(OH)D collection time, and the purpose of outcomes may contribute to generate different outcomes.
SUMMARY
Vitamin D has been known as an important hormone in the skeletal & non-skeletal system during pregnancy, but its precise role in pregnancy is still unclear. Although the researches for association between vitamin D and pregnancy outcomes are not still enough to reach consistent results, vitamin D should not be interpreted to have an insignificant effect on pregnancy until large randomized, controlled, prospective studies elucidate the relationship between maternal vitamin D and GDM and fetal growth, and obtain definitive conclusions.
Notes
No potential conflict of interest relevant to this article was reported.