Relationship between Bone Mineral Density and Spinal Muscle Area in Magnetic Resonance Imaging
Article information
Abstract
Background
Bone mineral density (BMD) is known to have a positive correlation with lean body mass. Several studies have also reported the positive correlation between muscle power and BMD. From this point of view, we hypothesized BMD of lumbar spine to have a positive correlation with muscle mass.
Methods
Seventy-nine female patients aged between 60 and 75 years old and who underwent magnetic resonance imaging (MRI) and BMD studies were included. Muscle mass in spine MRI was defined by the sum of the average muscle area of three axial images for each disc level. Lumbosacral muscle is the sum of paraspinal muscle and psoas muscle.
Results
In correlation analysis, paraspinal muscle mass showed positive correlation with BMD of lumbar spine. Lumbosacral muscle mass showed positive correlation with BMD of trochanteric area of the femur. However, BMD of other area showed no significant correlation with muscle mass.
Conclusions
Therefore, postmenopausal women older than 60 years with a well developed spine muscle mass, have a high BMD.
INTRODUCTION
Bone mineral density (BMD) is influenced by genetic factors like as body fat content, lean body mass and muscle power.[12] BMD not only has correlation with genetic factors but with correctable factors such as body fat, lean body weight and muscle power.[34] Also an individual with low BMD has a higher risk for fracture.[567] Therefore, identifying correctable factors which may influence BMD can aid in establishing preventive measures for osteoporotic fractures.
Lean body mass has been identified as one factor which determines BMD.[891011] Also the large paraspinal mass around the lumbar area is a factor relevant for the high BMD around the lumbar and proximal femur.[12131415] Since paraspinal muscles directly act upon the lumbar spine and the psoas muscle originates from the lumbar and inserts on the femur, we can hypothesize that the paraspinal and psoas muscle area has a correlation to femur and lumbar BMD. For postmenopausal women, fat density has a positive correlation to BMD.[111617] Therefore, we can hypothesize that a thick adipose layer posterior to the spinous process will result in increased BMD. Also, there is a study which shows mechanical protection of the proximal femur results in reduction of femur fractures.[18] Since there are no muscles posterior to the spinous process only the adipose layer can had mechanical protective properties, therefore we can predict that the thickness of the adipose layer is an important prognostic factor in lumbar osteoporotic fractures.
Through dual energy X-ray absorptiometry (DXA), the muscle mass and the net muscle mass of the extremities can be calculated by calculating the lean body mass and the amount of soft tissue, respectively.[19] However, the mass of the hip muscles and the paraspinal muscles cannot be calculated using this method.[2021] Also, DXA is not an appropriate method for calculating fat mass for the trunk area where the lumbar is situated.[22] However, through magnetic resonance imaging (MRI) used for evaluation of the spine, we can observe the axial plane of the muscles and fat tissues therefore is the most accurate study for evaluating the muscle and fat tissue mass in the body.[2223] This study evaluated the correlation between paraspinal lumbar muscle area and fat thickness to BMD of the femur.
METHODS
1. Study population
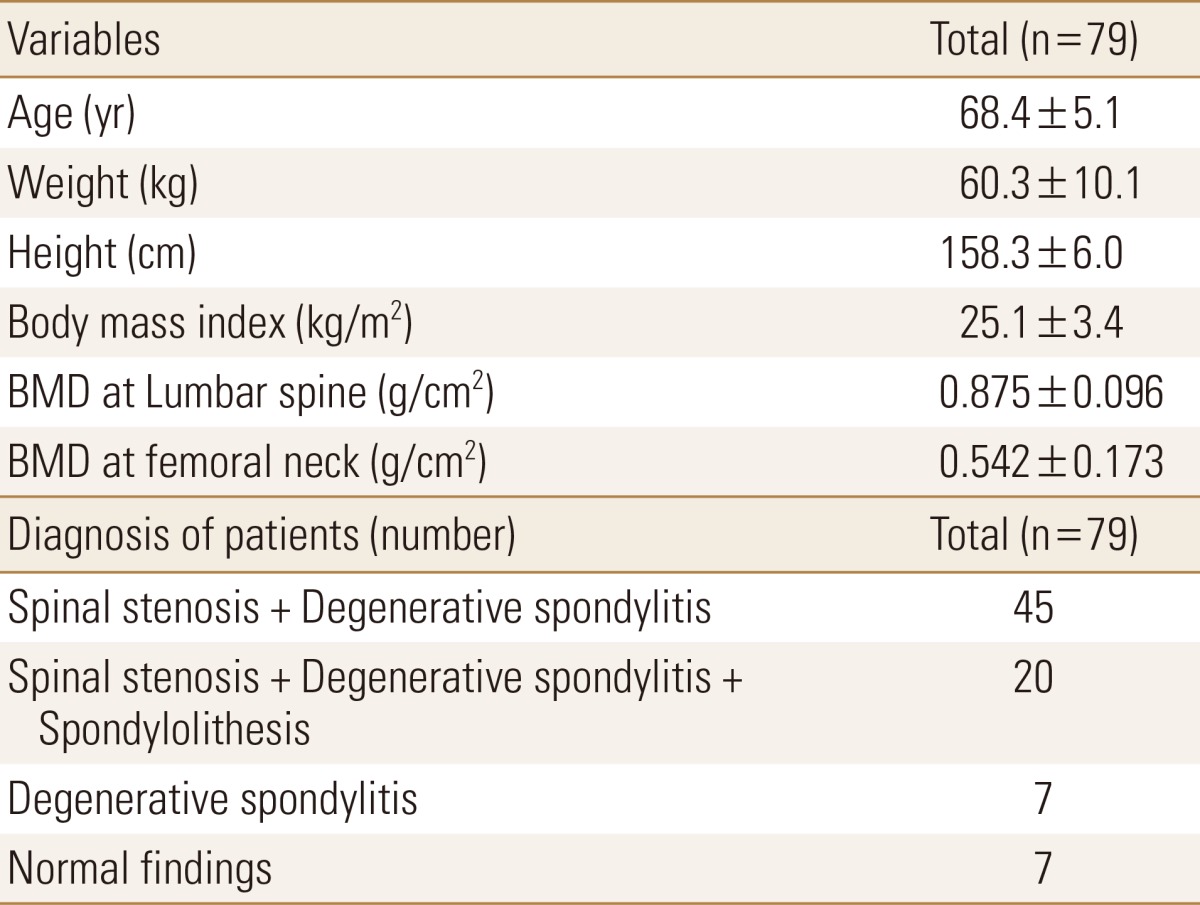
Women between ages 60 to 75 who visited the orthopedic department of Yonsei University Medical Center during January 2006 to December 2006 with lower back pain and who underwent plain x-ray and MRI of lumbar spine and BMD of lumber and femur were included in the study. After retrospective examination of out patient medical records, patients who had lumbar surgical procedures and compression fractures were excluded and as a result 79 patients were included in the study. The diagnosis of the patients were as follows. Forty-five patients had spinal stenosis with degenerative spondylitis. Twenty patients had spinal stenosis, spondylolisthesis and degenerative spondylitis. Seven patients had only degenerative spondylitis and 7 patients had no specific findings. Table 1 Shows baseline characteristics of study population.
2. Study method
For BMD all patients in the study used DXA method (Hologic QDR-4500A, version 12.4; Hologic Inc., Bedford, MA, USA). For every BMD examination was calculated after confirming a coefficient variation of less than 1.0% by using the lumbar phantom model. T1 weighted MRI axial images were obtained (1.5 T, TR 450 ms, TE 17 ms, spin echo).
3. Measured value
Three dimensional measurement of the lumbar muscle mass would have been ideal, however it is difficult to calculate the muscle volume from axial images parallel to intervertebral disc space which can change according to the position of the patient. However by obtaining the total sum of the area of each axial image a similar value to the muscle volume can be depicted. We named the sum of the paraspinal muscle and the psoas muscle area as the lumbosacral muscle area and defined this as the lumbar muscle mass. The below equation simplifies this definition.
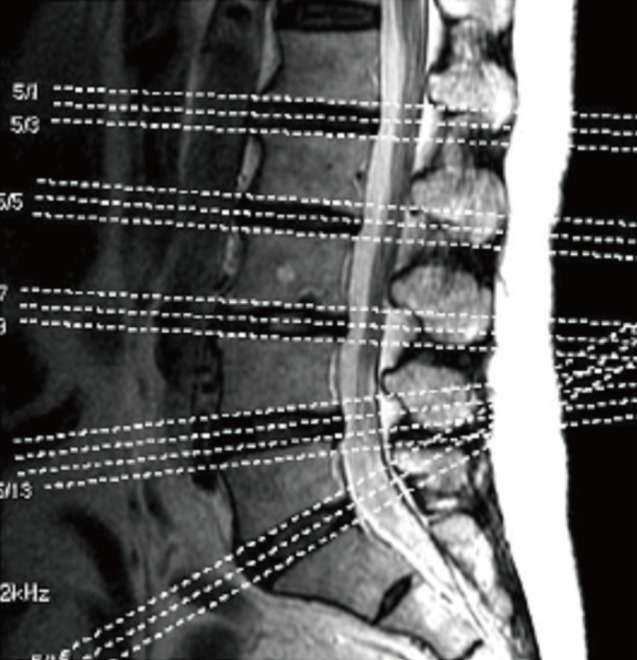
Muscle area was calculated by drawing a boundary around each paraspinal and psoas muscle using the picture archiving and communication system (PACS; Centricity, GE Healthcare, Piscataway, NJ, USA). Muscle area was measured for each interval between L5/S1, L4/5, and L3/4. Below is an explanation of how paraspinal muscle area between L3/4 interval was calculated. First one axial image correlating to one of the lines in Figure 1 (sagittal image) was chosen (Fig. 2) and by using the region of interest (ROI) function on the PACS system the boundary was drawn for the paraspinal and psoas muscle. After a folium is formed through this method the PACS systems calculated the area inside the folium in mm2 units. The 2 folium shown at the bottom of Figure 2, i.e. the sum of the areas of the paraspinal muscles becomes the area of the area of the paraspinal muscle in this image. To reduce the error two other cuts from L3/4 interval was calculated by the same method and the average of the 3 calculated values was recorded as the average area of the paraspinal muscles of L3/4. This final value was added to the paraspinal area of L4/5 and L5/S1, calculated by the same method. The sum of these three values was defined as the area of the paraspinal muscle. Psoas muscle area was calculated using the same methodology. The below equation gives a schematic explanation.
Paraspinal muscle area of one L3/4 axial cut (cm2) = Rt paraspinal muscle area + Lt paraspinal muscle area
Paraspinal muscle area of L3/4 interval (cm2) = Average area of 3 axial cuts of L3/4 interval
Paraspinal muscle area (cm2) = L3/4 paraspinal area + L4/5 paraspinal area + L5/S1 paraspinal area
For subcutaneous fat, the shortest distance between the skin and spinous process was used due to the absence of a defined boundary of fat tissue as seen in muscle fascia. As seen in Figure 2 an imaginary line connecting the shortest distance from the spinous process to the skin was used to calculate the thickness of the fat tissue. The average thickness of 3 axial cuts in each spinal level was used as the thickness of fat at each layer and the sum of these values was used to obtain the total thickness of fat tissue for the lumbar area.
Fat thickness of L3/4 (mm) = Average thickness of 3 axial cuts of L3/4
Total Fat thickness (mm) = L3/4 fat thickness + L4/5 fat thickness + L5/S1 fat thickness
For BMD of lumbar spine, the total bone mineral content (BMC) from L1 to L4 was divided by the total area.
For muscle area and fat thickness on MRI the observer either selected a ROI with the mouse or measured the length. Therefore an error may develop when the observer judges the boundary or sets a ROI on basis of signal density. To evaluate the intra-observer degree of error of area evaluation with MRI the coefficient of variation was calculated. This coefficient was obtained by repeating 10 calculations daily for 10 days for 5 randomly selected patients per day. The cardiovascular for paraspinal muscle area was 3.6%, psoas muscle area 1.9% and lumbosacral area 2.8%. Also, when performing the BMD examination the patients check their height and body weight. The relationship between weight, height and body mass index (BMI; weight/height2) to BMD and muscle mass of the patient was analyzed.
4. Statistical analysis
The relationship between the calculated values was analyzed by using Pearson's correlation coefficient. Also, the Student's t-test was used for muscle area which showed a positive correlation with BMD on Pearson's correlation analysis. The difference between muscle area between osteoporotic group (T-score <-2.5) and non-osteoporotic group was evaluated using independent sample t-test. To verify the factors affecting BMD, multiple regression analysis was performed on a model made based on factors previous reported to affect BMD. The SPSS 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical programming. A P-value less than 0.05% was considered to have statistical significance.
RESULTS
The average and standard deviation for lumbar muscle mass calculated by lumbar MRI was calculated. Paraspinal muscle area was 73.34±15.30 cm2 psoas muscle area 32.34±7.30 cm2 and lumbosacral muscle area 105.69±19.01 cm2 (Table 2). The distribution of each calculated value is shown in Figure 3.
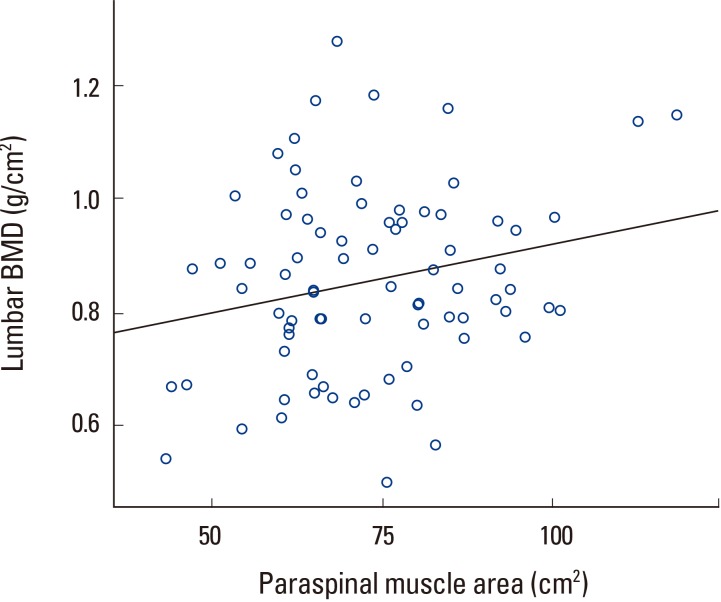
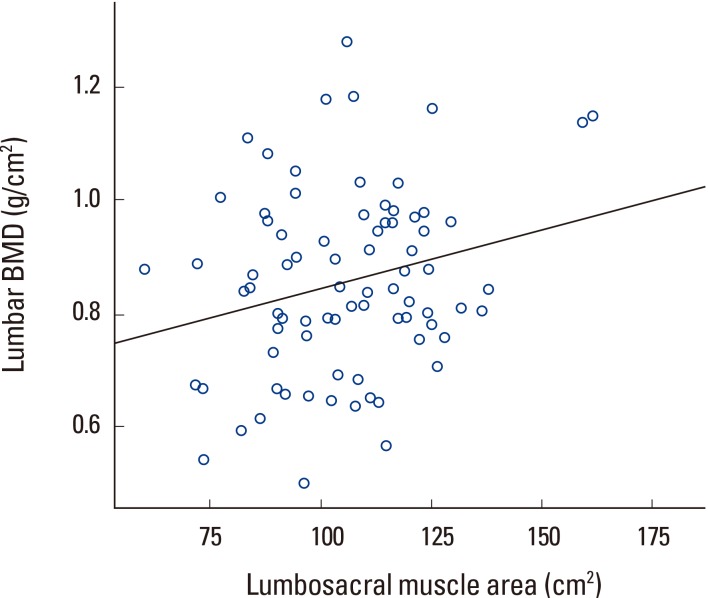
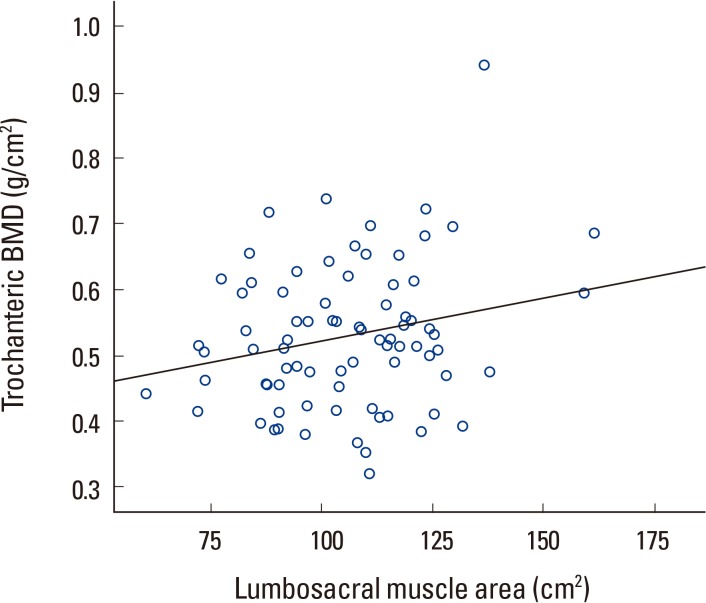
Correlation analysis showed that lumbar BMD had no correlation with psoas muscle area but showed statistically significant correlation with paraspinal muscle and lumbosacral muscle area (Table 3, Fig. 4, 5). However, lumbosacral muscle area is the sum of paraspinal and psoas muscle area and is around twice that of paraspinal and psoas area. Therefore, the correlation between lumbosacral muscle area and BMD can be seen as a result of the correlation between paraspinal muscle area and lumbar BMD.

Pearson correlation coefficient between bone mineral density and lumbar muscle mass and fat thickness
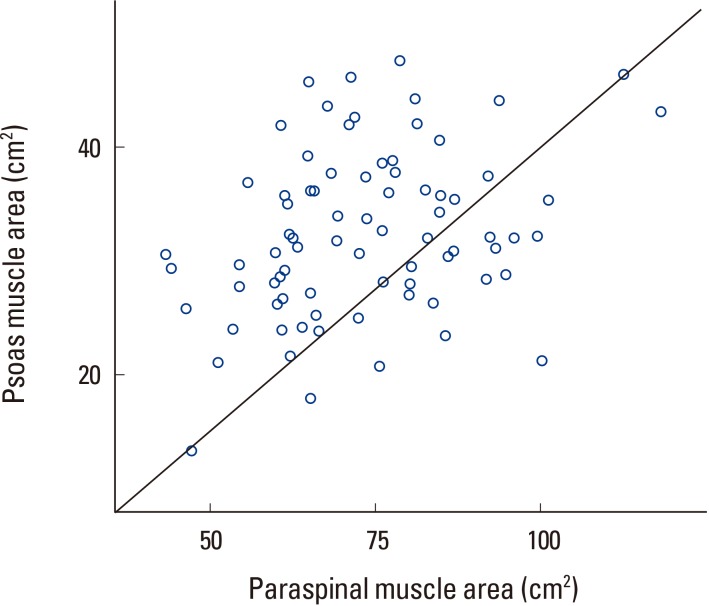
Trochanteric BMD had statistically significant correlation with lumbosacral muscle area but no correlation with paraspinal muscle and psoas muscle area (Table 3, Fig. 6). In other words, each individual area had no correlation, but the sum of the areas showed significant correlation to trochanteric BMD. Correlation analysis of paraspinal and psoas muscle showed a positive correlation with a P-value less than 0.05 (Fig. 7).
Femur neck BMD showed no significant correlation with paraspinal, psoas and lumbosacral muscle area. The total femur BMD also showed no correlation with paraspinal, psoas and lumbosacral muscle area. Correlation analysis results showed no correlation between total fat thickness to lumbar and entire femur BMD (Table 3).
Body weight showed a statistically significant positive correlation with all BMDs, all muscle area and fat thickness. Height showed no significant correlation with any of the factors and age showed a significant negative correlation with femur BMD and a positive correlation with fat content. Lean body weight showed a significant positive correlation with all factors similar to that of body weight (Table 4).
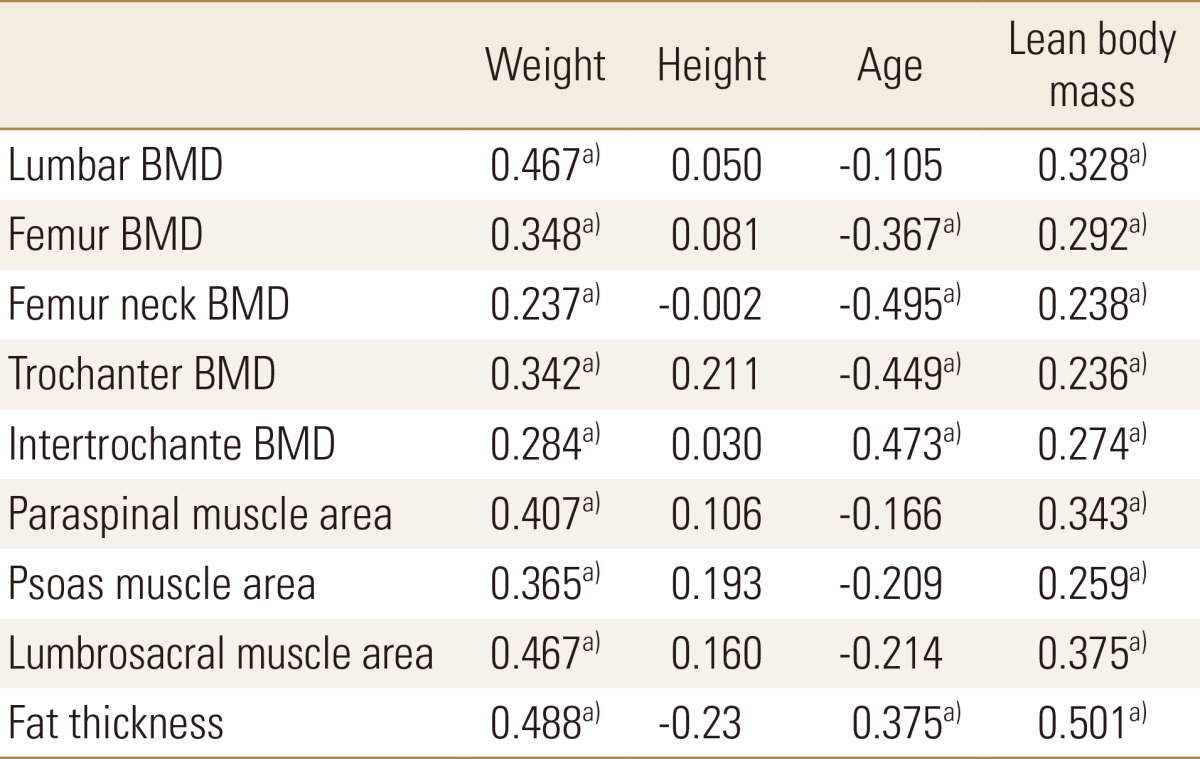
Pearson correlation coefficient of bone mineral density, muscle area and fat thickness to weight, height, age and lean body mass
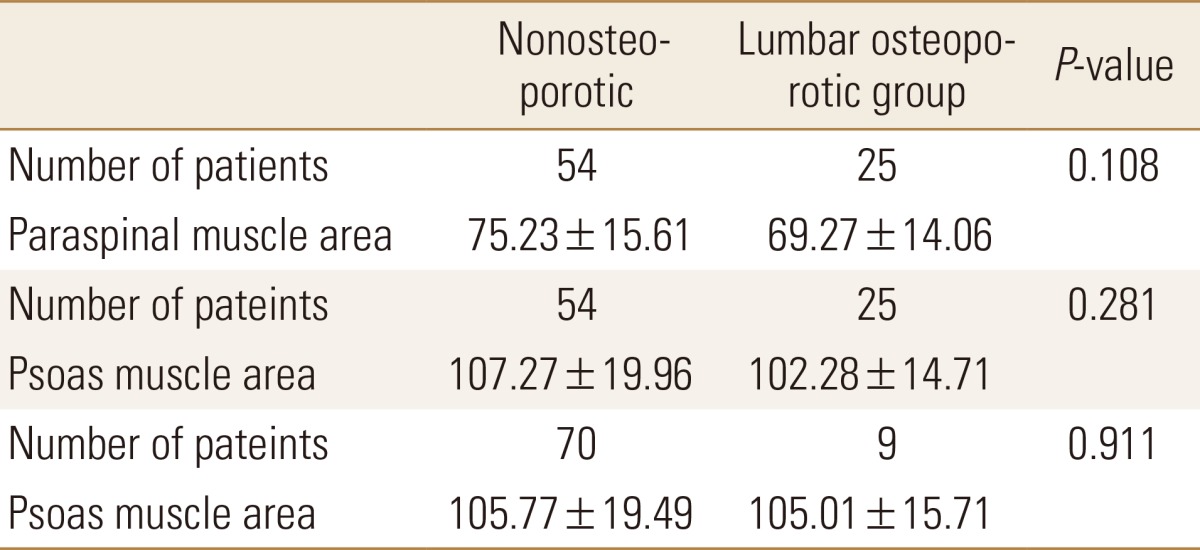
Using the above data comparison of paraspinal muscle area and psoas muscle area between lumbar osteoporotic and nonosteoporotic group, psoas muscle area between trochanter osteoporotic and nonosteoporotic group was performed using independent samples t-test. However, analysis showed no statistical difference between osteoporotic and nonosteoporotic groups in terms of muscle area (Table 5).
Body weight, lean body mass, paraspinal muscle area, psoas muscle area, age and fat thickness which affects BMD at each location of the femur were analyzed through multiple regression analysis. Body weight and lean body mass were individually significant factors, while paraspinal muscle area, psoas muscle area and muscle thickness showed no significance.
DISCUSSION
It had been reported that BMD distribution is influenced by mechanical loading and this has been proven in a study on athletes.[2425] It is true mechanical loading due to gravity can be a result of both lean body mass and fat content.[26] However, the force generated by muscle contraction is the strongest force applied to bone.[272829] Also is known that muscle contraction force can be used to predict BMD at various locations.[12131415] This study shows the relationship between paraspinal muscle area and lumbar BMD. Paraspinal muscle and psoas muscle area showed significant correlation, however, psoas muscle area showed no significant correlation to lumbar and femur BMD. This is due to a weaker correlation between lumbar muscle area to BMD than reports on the stronger correlation between lean body mass to BMD using dual energy absorptiometry studies.[113031]
Spinal MRI axial images are obtained by the position of the intervertebral sagittal image in an individual patient. Therefore, the lumbosacral area obtained even in the same individual can change according to the position of the patient during the imaging study. This effect is prominent in the psoas muscles located anterior to spinal canal than in the paraspinal muscles. This effect may be the reason for the weak correlation between psoas muscle area to BMD.
Conversion of androstenedione to estrogen usually occurs in adipose cells.[32] This estrogen reduces reformation of bone.[33] Testosterone directly facilitates bone formation through androgen receptors and indirectly inhibits bone resorption by converting to androstenedione. The conversion of such molecules at adipose tissue is the reason for the evident correlation between fat content and BMD in women.[343536] This correlation is known to be more evident in postmenopausal women.[37] However, in our study on postmenopausal women, there was no correlation between fat thickness posterior to spinous process and BMD. This is probably due to the reason that 3 dimensional volume of fat tissue was not truly represented by 2 dimensional fat thickness alone. As was shown in many other studies, BMD had significant correlation to body weight and lean body mass.
Paraspinal muscle area had correlation with lumbar BMD and lumbosacral muscle area had correlation with femur trochanter BMD. Therefore, it was confirmed that there is a high possibility that lumbosacral muscle area has a significant effect on lumbar and femur osteoporotic fracture.
With further study of a larger cohort and longer follow up with inclusion of patients in this study it will be possible to clarify the relation of lumbar muscle area seen on MRI to lumbar and femur osteoporotic fracture.
CONCLUSION
The BMD is increased in postmenopausal women over 60 years of age with a relatively well developed paraspinal muscle.
Notes
No potential conflict of interest relevant to this article was reported.